Extracting terpene drugs from plants is difficult and wasteful, so pharma companies are looking to biosynthesis, as Emiliano Feresin discovers

In August 2014, the pharmaceutical company Sanofi delivered to Africa the first stock of artemisinin-based malaria treatments produced in fermentation tanks by genetically engineered yeast. It was the result of 14 years of research led by Jay Keasling, a synthetic biologist at the University of California, Berkeley in the US. He pursued the idea of finding an alternative to plant extraction for the production of the potent terpene artemisinin. ‘It was an incredible learning experience that will help producing new drugs from terpenes,’ says Keasling.
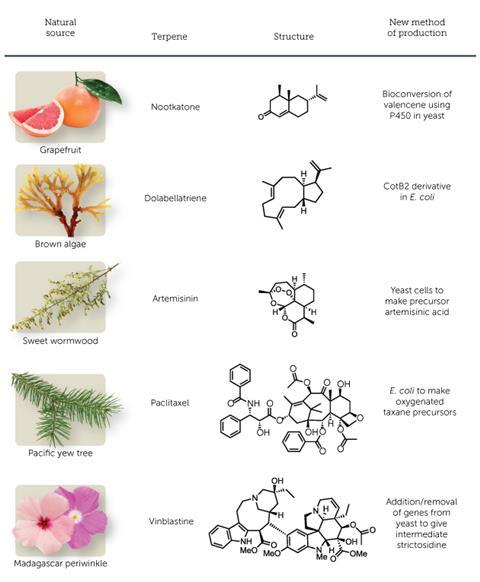
With more than 75,000 molecules identified to date, terpenes are the largest and most diverse class of natural compounds. Multiple isoprene units are linked together to form their cyclic or acyclic carbon skeletons, and chemists use the number of carbon atoms to classify them. Terpenes are all around us: plants, insects and fungi generate small amounts of these secondary metabolites to attract or repel insects, or as signal molecules. Limonene, a monoterpene with a 10-carbon skeleton, and nootkatone, a 15C sesquiterpene, are found in lemon and grapefruit peel respectively and are responsible for their distinctive smell; menthol and camphor are other everyday monoterpenes. Simple monoterpenes and sesquiterpenes are volatile and smell good, and have been attracting the flavour and fragrance industry for a long time.
Complex terpenes with cyclic structures are not volatile and often have interesting medical properties. ‘Natural products and terpenes in particular show a very good record in providing leads to pharmaceutical products, hence there is a high probability to find bioactive molecules in this class,’ says Gregory Stephanopoulos, chemical engineer at the Massachusetts Institute of Technology in the US. Popular examples of terpene drugs are artemisinin (a tetracyclic sesquiterpene), paclitaxel (a potent 20C diterpene anticancer drug marketed as Taxol), and vinblastine (a monoterpene-derived anticancer drug, approved as Velban).
Stairways to terpenes
Historically, plant extraction has been the preferred approach for terpenes production. ‘Extraction can be extremely cost inefficient and unsustainable, while the supply is affected by plant disease, weather conditions and international trade-related factors,’ says Meraldo Antonio, an analyst at Lux Research, a consulting company in Boston, US. Synthesis comes into play only for some monoterpenes (such as limonene) and linear terpenes like beta-carotene (40C).
‘Stereoselective synthesis of terpene macrocylces is a major hurdle in the chemical synthesis of terpenes,’ says Thomas Brück, an expert in industrial biocatalysis at the Technical University of Munich, Germany. ‘A chemist would require 15–30 steps to obtain the stereochemical complexity of a diterpene macrocyclic core,’ he adds. Paclitaxel, for example, has 11 stereocenters.
The discovery and characterisation of numerous enzymes involved in terpene pathways and the recent advent of metabolic engineering have paved the way to the biosynthetic production of some terpenes using microbial cell hosts in fermentation tanks. The new technology is becoming a competitor to extraction. A handful of new companies, including Isobionics, Evolva and Oxford Biotrans, has recently entered the market offering the industrial biosynthesis of terpene-based flavours and fragrances. They all produce the sesquiterpenes nootkatone and valencene – a citrus-like aroma compound found in the peel of Valencia oranges, but have other terpenes in the pipeline. Evolva also plans to sell the popular diterpene sweetener stevia by 2016. Larger chemical companies such as DSM, DuPont and BASF have R&D units dedicated to the biosynthesis of new natural molecules.
A chemist would require 15–30 steps to obtain the stereocentres in a diterpene macrocycle
The pharmaceutical industry, on the other hand, lags behind in the biosynthetic production of terpene-based drugs. So far, artemisinin is the only terpene being produced biosynthetically. Sanofi launched large-scale production in 2013, after Amyris, the spin-off company started by Keasling, optimised the pathway and the development of the production process.
One of the reasons for pharma’s slow reaction towards the biosynthesis of bioactive terpenes is technical. ‘An efficient biosynthetic production of complex terpenes is challenging to establish as it often involves numerous steps, all which need to be fully optimised before a full-fledged industrial synthesis can commence,’ says Antonio. ‘As the knowledge on synthetic biology continues to develop, the synthetic version of complex terpenes will eventually catch up.’ Some of the more advanced labs in the world are currently trying to solve the most intriguing pathways that lead to bioactive terpenes.
Mine the gap
The natural biosynthesis of cyclic terpenes is a complicated process, usually involving more than 20–30 enzyme-catalyzed reactions. The upstream section is now fully understood: Microbial enzymes convert simple monomeric sugars to the universal terpene precursors IPP (isopentyl pyrophosphate) and its isomer DMAPP (dimethylallyl pyrophosphate). In the downstream pathway, IPP and DMAPP are condensed into group-specific polyprenyl diphosphate intermediates. Product-specific cyclases first generate a cyclic terpene skeleton, and then oxidoreductases and functionalising enzymes lead to the final terpene with its peculiar bioactivity.
But the product-specific downstream enzymes are elusive molecules. ‘It’s very difficult to identify the target cyclases in the genome, because plants produce many terpenes and cyclases show very low amino acid sequence identity to already known enzymes,’ says Bernhard Loll, a structural biologist from the Free University of Berlin in Germany. These enzymes are usually found by genome-mining techniques, and their function can only be inferred through an enzyme assay, analyzing the terpene products.
A pandora’s box for new drugs
In 2013 Loll joined forces with Brück to elucidate the metabolic pathway of diterpene macrocycles. In June 2014 the Berlin–Munich collaboration published the first crystal structure1 of a bacterial diterpene cyclase, CotB2, with a resolution of 1.64Å. CotB2 catalyses the single-step cyclisation of geranylgeranyl diphosphate (GGPP) to generate cyclooctat-9-en-7-ol, a molecule just two steps away from cyclooctatin, a diterpene with anti-inflammatory activity. Cyclooctatin lacks some severe side effects compared to existing blockbuster drugs aspirin or ibuprofen. The German scientists also proposed a mechanism for the carbocation migration during the cyclisation.
‘Cyclases catalyse the most complex reactions in biology. Their hallmark is the generation and propagation of reactive carbocation intermediates to drive carbon–carbon bond formation,’ says David Christianson, an expert in terpene biosynthesis from the University of Pennsylvania, US, who was not involved in the CotB2 research. Christianson’s group has solved the three-dimensional crystal structures of 12 of the 21 terpene cyclases published so far. In 2011, they solved the first x-ray crystal structure of a diterpene cyclase, the taxadiene cyclase (TXS) that, after cyclisation of GGPP, catalyses the formation of the 11-stereocenter macrocyclic core of the potent anticancer paclitaxel.2
Cyclases catalyse the most complex reactions in biology
Loll and Brück went further than solving the crystal structure of the CotB2 and proposing the mechanism. ‘If you know precisely how the enzyme works and which intermediates are formed, then you can infer which amino-acid residues are relevant and predict what kind of cyclic product you get upon mutagenesis,’ explains Ronja Janke, a PhD student in Loll’s lab. They performed structure-guided mutagenesis into the now-known active site of the enzyme to see if they could get products different from the natural one.
‘The mutations led to new bioactive compounds that astonishingly have a defined stereochemistry and variable number of rings,’ says Loll. One compound, the bicyclic terpene dolabellatriene, has shown potential antibiotic activity against multidrug-resistant Staphylococcus aureus. Brück could even produce dolabellatriene with a concentration or titre of 1.7mg per litre of fermentation mixture in a recombinant E. coli system without any toxicity effect for the host microbe and recently achieved yields up to 8.0mg per litre. A main advantage of CotB2 is its ability to give pure compounds: ‘Similar mutations in other cyclases could result in complex product mixtures,’ says Loll.
The consortium is now looking to complete the cyclooctatin pathway with the two remaining cytochromes P450 and to optimise it in fermentation tanks. They are also working on other cyclases and potential promising diterpenes. ‘Complex diterpenes are the new pandora’s box for drugs,’ says Brück, who collects soft corals during his research expeditions in search for new terpenes.
But other terpenes may require more than just the two functionalisation steps of CotB2. The identification of functionalising enzymes like P450 is another major subject of intense studies, because the required enzyme repertoire is not available yet, says Brück. Still, for some cases, getting the macrocyclic terpene intermediate may be enough. ‘We can now offer these complex scaffolds to chemists, they can use them to synthesise other compounds,’ says Janke.
The yeast we can do
The idea of getting halfway through a pathway to an intermediate and then to apply chemical synthesis for further derivatisation is not completely new. ‘Many intermediates have similar structures to the final terpene,’ says Stephanopoulos. ‘Hence if we can derivatise more intermediates chemically, the probability to get some molecules with pharmaceutical properties is significantly higher.’
Stephanopoulos’ group has been at the forefront in the quest for a biosynthetic production of paclitaxel. The molecule was first extracted from the bark of the Pacific Yew tree, and initially two to four fully grown trees were required to cure one patient. Today’s strategy also relies on the extraction of the intermediate baccatin III from the European Yew and subsequent synthesis as well as plant cell fermentation technology.
In 2010, Stephanopoulos’ group worked with Singaporean researchers to reproduce the first cyclic intermediate of the path, taxadiene, in E. coli and the subsequent oxidised intermediate taxadien-5a-ol.3 They engineered the microbe to contain four bacterial genes that lead to the universal terpene precursors IPP/DMAPP, as well as two additional plant genes that code for the downstream enzymes – the condensation enzyme GGPP synthase and the taxadiene cyclase. They screened 32 different strains for the best combination that allowed for the least accumulation of toxic intermediates and finally obtained 1g of taxadiene per litre in the fermenter – 1000-fold higher than previously obtained in microbes. Further addition of the cytochrome oxidase P450 allowed the researchers to get taxadien-5a-ol for the first time in microbes at a concentration of 58mg per litre.
Once they had obtained the intermediates, the MIT researchers began optimising their production. ‘One challenge is to put together a pathway, the next is to optimise it to get a significant amount of the molecule,’ says Stephanopoulos. He tried a co-culture of two microbes. ‘Based on the idea that yeast is best for hydroxylation, we made E. coli produce taxadiene and S. cerevisiae perform the oxidase reaction,’ he says. This modular approach was possible given that taxadiene can cross cell membranes and diffuse into the yeast for further steps. In their first attempt, they used glucose as a carbon source for both microbes, but soon realised that the ethanol produced by the yeast was inhibiting E. coli productivity. Hence they switched to a so-called mutualistic interaction: introducing xylose as carbon source lead to the bacterium producing acetate, which the yeast could use as a carbon source. In this way they were able to produce oxygenated taxanes at 33mg per litre, including one important intermediate in the paclitaxel synthesis, which was identified as a monoacetylated deoxygenated taxane.4
The road to vinblastine
Diterpenes are not the sole pool in which to look for new drug candidates. Sarah O’Connor, a biochemist at the John Innes Centre in Norwich, UK, studies iridoids, complex bicyclic monoterpenes, and their derivatives called monoterpene indole alkaloids (MIAs). There are around 3000 known MIA molecules and all of them are biosynthesised via one specific iridoid called secologanin. Many monoterpene alkaloids show interesting bioactivities, like the approved anticancer drugs vincristine and vinblastine. Vinblastine is prescribed against breast, testicular and bladder cancers, but to produce 1g of the compound it’s necessary to harvest about 500kg of leaves from Catharanthus roseus (Madagascar periwinkle; see main image above). The plant alone produces more than 100 MIAs and it’s one of the most studied medicinal plants.

‘It would be very exciting to be able to reconstitute the full vinblastine pathway, but finding the enzymes that comprise the plant pathway is the main hurdle,’ says O’Connor. Her group investigates both the iridoid pathway to secologanin and the subsequent pathway to vinblastine in C. roseus. They recently identified the cyclase that generates the iridoid scaffold.5 Starting from available plant gene expression data, they restricted the candidates’ pool to those having similar expression profiles to known biosynthetic genes in the pathway. Their cyclase candidate (ISY) was expressed in E. coli and gave a result in contrast to the general cyclisation mechanism via carbocations. ‘In iridoids, geranyl diphosphate is first oxidised into 8-oxogeranial, which in turn undergoes cyclisation via a reductive type mechanism,’ explains O’Connor.
Including ISY, the path to vinblastine biosynthesis comprises more than 20 genes. ‘We still need at least four or five to complete the pathway,’ admits O’Connor. The missing genes are all downstream of strictosidine, the intermediate for all known MIAs that is generated by reaction of the iridoid secologanin and the indole triptophan. O’Connor is now looking for shortcuts. ‘Some plants have shown that pathway genes can be physically clustered; if this happens in C. roseus it would facilitate the identification of the missing enzymes,’ says O’Connor. She recently assembled a draft plant genome using sequencing by-synthesis and then looked at the genes involved in the MIA pathway. ‘We’re still mining the data, but so far we see just small partial clusters,’ she says.
In the meantime, the Norwich group reconstituted in yeast the pathway halfway to strictosidine. Since all the enzymes are known, they integrated them into S. cerevisiae and made it stable. ‘Yeast is not very good in producing monoterpenes, so, learning from the previous work on artemisinin and other terpenes, we over-expressed the few genes that comprise that part of the pathway,’ explains O’Connor. Upon introducing 21 new genes and deleting three genes in the yeast genome, she finally got 500mg of strictosidine per litre.6 ‘We were very pleased with the result, but for a commercial application we would have to improve this yield by several orders of magnitude,’ concludes O’Connor.
At a crossroads
Researchers are very optimistic over the appeal of the new biosynthetic approach to drug development. ‘I can guarantee that if someone engineers a pathway for taxol, before you know it, it is going to be in production,’ says Stephanopoulos. He foresees that with the right funding the next product could be on the market in two or three years. Brück is more cautious on the time scale: ‘The next product is 7–10 years away,’ he says. ‘The biosynthesis of artemisinin took more than 110 man-years, an excess of €50 million over 12 years; for a diterpene you can probably double these figures.’ Funding, however, does not guarantee a smooth path. Scaling up to pharma production requires concentrations on the order of grams per litre, a goal that needs careful balancing of every step in the pathway, warns Keasling.
There have been companies that have gone down that road; none of them really exist now
But even if basic research solves the riddle of getting the terpenes at reasonable titres, it’s unclear if the pharma industry is interested in investing in process optimisation and the scaling-up step. ‘At least in the near future, the industry is more interested in the biosynthetic production of simple terpenes,’ says Antonio from Lux Research. More than the technical hurdles it’s the need for cost reduction that drives one industry instead of the other. ‘The food, perfumery and the consumer products industries are under pressure to find cheaper alternative routes to their ingredients, hence their interest for biosynthesis of simple terpenes,’ he says. The pharmaceutical industry finds itself in the opposite situation, because more people are willing to pay a significant sum of money to obtain the most efficacious medicine, the pressure for that industry to reduce its costs is not as great, adds Antonio.
‘The pharmaceutical industry is currently more interested in sophisticated biological approaches to cure diseases; small molecules are not playing a fundamental role,’ says Neil Goldsmith, chief executive of Evolva. Indeed, vinblastine, paclitaxel and artemisinin are the only terpene-based drugs that have gone through clinical trials or tests in the years from 2000–2010. Evolva’s initial mission was to produce new pharmaceuticals, but the lack of interest redirected it towards making existing molecules in better ways. Goldsmith still foresees a lot of potential for the alternative production of natural drugs already in use, but less for new natural product variants, which would have the additional hurdle of testing. ‘There have been some companies that have gone down that road; none of them really exist now,’ he says.
Loll is fascinated by CotB2 plasticity and wants to develop the microbial production platform inside his spin-off company. But terpene pathways can lead to so many different compounds that he won’t stick to pharma-related pathways. ‘I’d modify our platform to produce high value ingredients for the food or flavour/fragrance industry as well,’ he says.
Emiliano Feresin is a science writer based in Berlin, Germany.
References
1 R Janke et al, Acta Crystallogr. Sect. D, 2014, 70, 1528 (DOI: 10.1107/s1399004714005513)
2 M Köksal et al, Nature, 2011, 469, 116 (DOI: 10.1038/nature09628)
3 P K Ajikumar et al, Science, 2010, 330, 70 (DOI: 10.1126/science.1191652)
4 K Zhou et al, Nat. Biotechnol., 2015, 33, 377 (DOI: 10.1038/nbt.3095)
5 F Geu-Flores et al, Nature, 2012, 492, 138 (DOI: 10.1038/nature11692)
6 S Brown et al, Proc. Natl Acad. Sci. USA, 2015, 112, 3205 (DOI: 10.1073/pnas.1423555112)









No comments yet