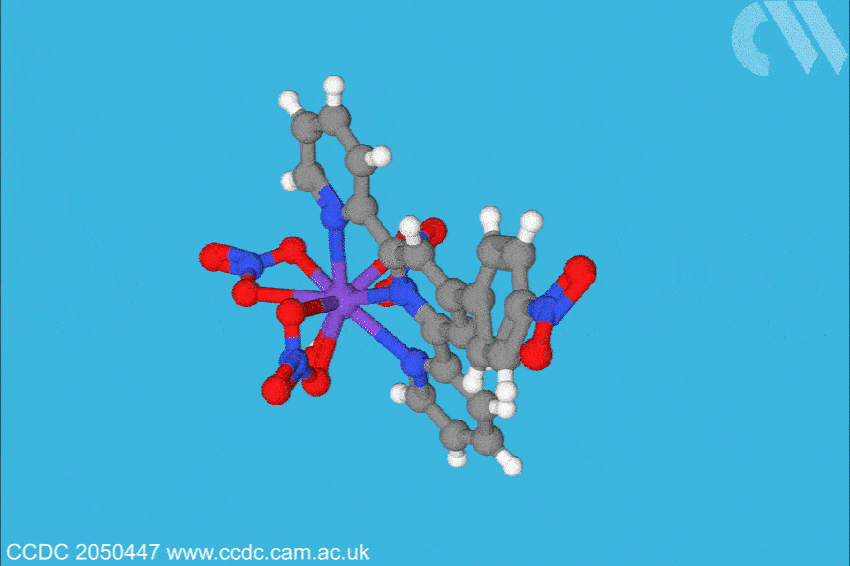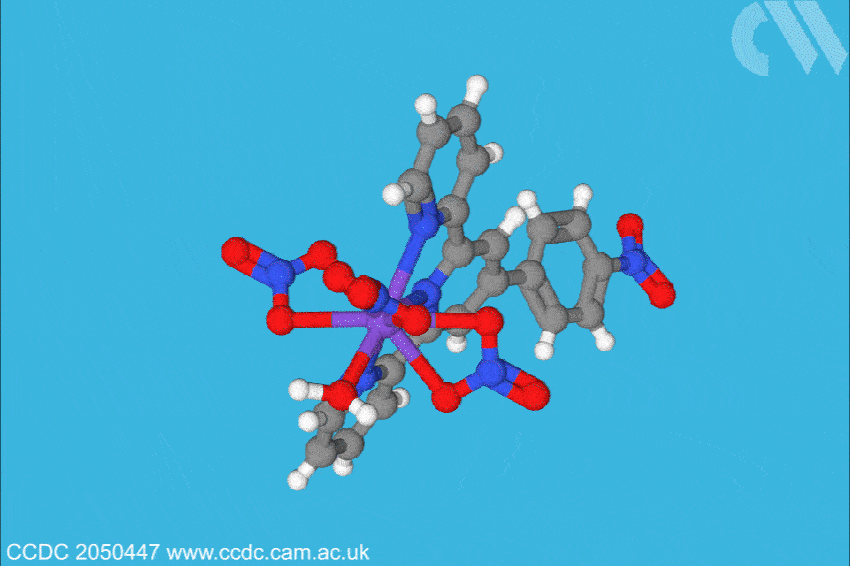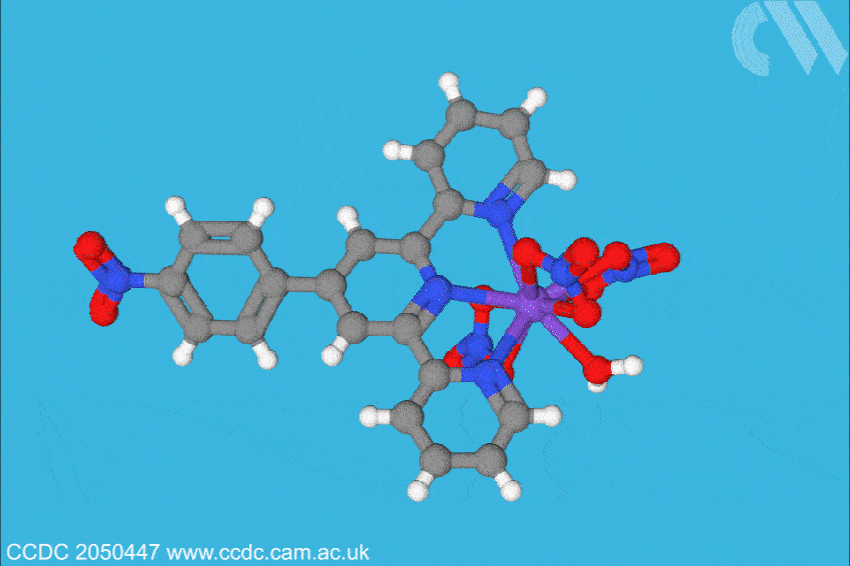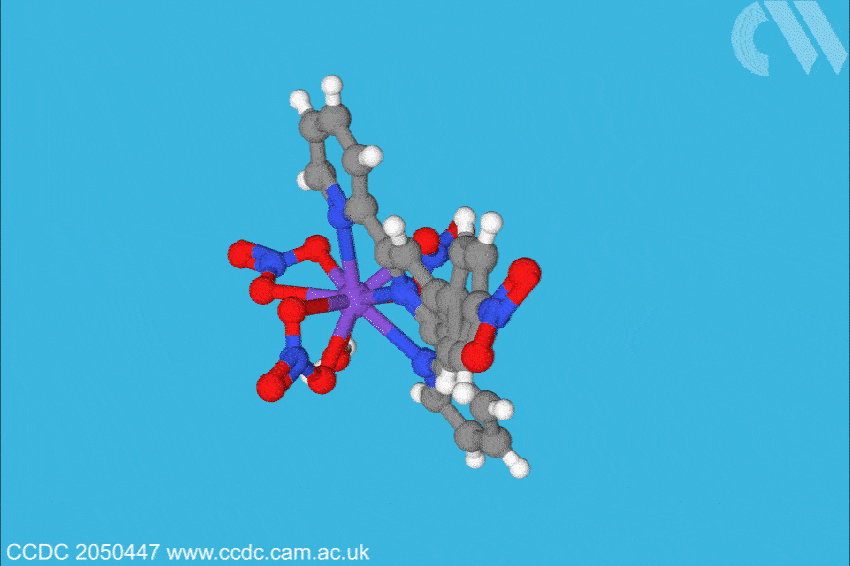
A new berkelium complex has shown that highly polarised ligands can be used to target heavier actinides, potentially paving the way for selective recycling of radioactive elements.
Since its discovery in December 1949, berkelium, element 97, has remained largely unexplored. The actinide element has no known applications, does not exist on Earth naturally and is expensive to synthesise, meaning only milligrams of the element are available globally. It is also highly radioactive, with its most common isotope, Bk-249, having a half-life of 330 days and decaying into californium, which causes a build-up of electric charge in samples. This, and previous research suggesting berkelium’s bonds are primarily ionic and similar to lanthanides, means it is usually overlooked in favour of more readily available elements.
Now, a team at Florida State University, US, has investigated what is only the sixth berkelium complex ever characterised, and found the situation may not be so straightforward. Led by Thomas Albrecht–Schönzart, the group bound Bk(III) to 4’-(4-nitrophenyl)-2,2’:6’,2”-terpyridine, a ligand with a large dipole. This, the team hoped, would polarise the berkelium electrons when the metal–ligand bonds were formed. The team also formed a metal–ligand complex with cerium, berkelium’s closest electro-chemical analogue, to compare any effects through structural analysis, spectroscopy and electrochemical analysis.
The team found the polarisation of the ligand shortened the metal–ligand bonds in the same plane more than expected, influencing the electron density. Crucially, the team also found signs that there had been a reduction in inter-electron repulsion and that 6p orbitals are hybridised with berkelium, and 5p orbitals, albeit to a lesser extent, with cerium. ‘The 6p orbitals are normally thought of as core orbitals not involved in bonding,’ explains Albrecht–Schönzart. ‘But here they are hybridising with the ligand orbitals, and this even creates a covalent bond with the water molecule trans to the ligand. The effects of the polarisation were much larger than anyone anticipated.’ In addition, electron paramagnetic resonance (EPR) spectra showed a rhombus-shaped signal when the cerium complex was investigated, suggesting the electronic environment around centre of the complex is highly anisotropic.
This unexpected complexity of the heavier actinides suggests berkelium may be far more interesting an element than its earliest investigations suggest. The custom-designed complex also acts as a proof-of-concept for creating highly polarised ligands that can achieve specific bond strengths with target molecules. This would allow scientists to custom-design ligands that target specific metals during radioactive recycling, allowing for the selective extraction of specific elements.
Conrad Goodwin, an actinide chemist at the University of Manchester, UK, says the paper is a ‘striking example to highlight the complexity’ of the heavier actinides. ‘It’s difficult to overstate how painful each advancement in this area – particularly with berkelium – can be both from a logistical standpoint, and also just material availability. This is a fascinating contribution to the fundamental coordination chemistry of elements beyond uranium.’
References
AN Gaiser et al, Nat. Commun., 2021, 12, 7230 (DOI: 10.1038/s41467-021-27576-y)















No comments yet