The first biocatalytic route to produce a broad range of lactams – highly sought-after building blocks for drugs, including penicillin antibiotics – has been discovered. The work, which created an enzyme from an iron-containing muscle protein to catalyse lactam synthesis, could offer a simpler, cheaper and more efficient way to make them.
Lactams are ring-shaped amides, derived from amino alkanoic acids, that come in different forms depending on the number of atoms in their rings. Although a few organic synthesis strategies to make lactams already exist, a route via direct C–H amidation, which functionalises unreactive C–H bonds, could offer a more convenient and efficient alternative.
Such a route, however, has remained elusive. Only γ-lactams, which have five-membered rings, had been made via C–H amidation, but it required expensive, rare metal catalysts that were inefficient, used harsh oxidising reagents and produced chemical waste. Now, researchers in the US and China have found a new approach using an iron-based enzyme derived from the haem-complex in myoglobin, a protein found in muscles.
‘Our methodology significantly improves upon previous methods in that it enables the cyclisation of dioxazolone substrates by means of an inexpensive, renewable and non-toxic iron-based enzyme,’ says Rudi Fasan, who led the work, formerly at the University of Rochester, now at the University of Texas at Dallas, US. ‘This method offers excellent stereoselectivity, it is scalable and it has a particularly broad substrate scope, allowing for the synthesis of lactams of varying ring size.’
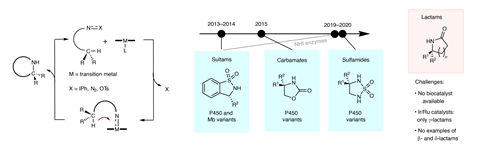
The team were inspired by previous work that had shown iron-based enzymes could catalyse reactions and introduce nitrogen into substrates. Wondering if this could work in lactam synthesis using dioxazolone reagents as nitrene precursors, the researchers set about testing various iron-containing enzymes and proteins for their activity producing γ-lactams via C–H amidation.
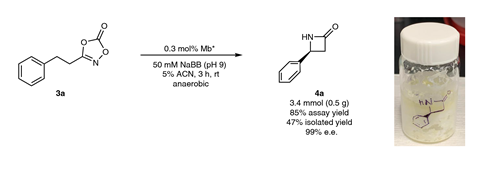
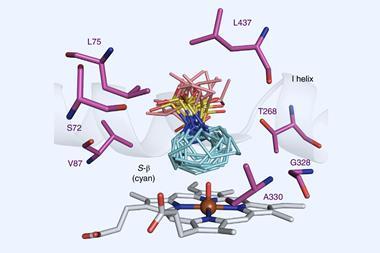
They arrived at an engineered myoglobin mutant, which the team had previously found had enhanced activity for nitrene transfer. Tests showed it reacted with dioxazolones to yield tiny yet detectable amounts of γ-lactam. Further mutations were then engineered in the active site of the mutant myoglobin to improve its performance.
‘Identifying an engineered variant and reaction conditions that maximise the productive reaction while minimising side reactions was critical,’ says Fasan. ‘While we have previously found that engineered myoglobin features an unusually broad substrate scope, its applicability across different lactam ring sizes was particularly remarkable.’
Results showed that the myoglobin catalyst could make a broad range of lactams – including β, γ and δ varieties – in good yields. β-lactams are particularly important for antibiotics. What’s more, the researchers demonstrated the simplicity and efficiency of the approach by producing two drug molecules – one an alkaloid natural product, the other a synthetic drug – in almost half the number of steps and in higher yields than other methods to make the same molecules.
‘The authors use stable, non-hazardous nitrene precursors derived from cheap and abundant carboxylic acids, so there is more chance that their method could find practical applications for scalable enzymatic synthesis of pharmaceuticals,’ says Jason Micklefield, a biocatalyst expert at the University of Manchester, UK. ‘They also show that their new enzymes are amenable to engineering, broadening the substrate scope and even switching the enantioselectivity.’
‘A key goal of our work is to make available to the synthetic community novel biocatalytic methods for stereoselective synthesis that are both enabling and practical,’ says Fasan. ‘Because of the technical simplicity of the present method, its high stereoselectivity and scalability, we expect it will represent an attractive method for lactam synthesis in both academic settings and in industry.’
References
S Roy et al, Nat. Catal., 2023, DOI: 10.1038/s41929-023-01068-2









No comments yet