Coiled polypeptides modified with redox-active molecules make for eco-friendly single-use batteries
The first battery made out of custom-built proteins has been created by US chemists. Instead of metal compounds, the battery’s electrodes contain polypeptides that have been altered to shuttle electrons with redox-active groups. Since proteins biodegrade, the technology could be a boon for single-use applications such as wearable sensors.
The work was presented at the American Chemical Society’s national meeting in San Diego, US.
According to consulting company GlobalData, battery storage capacity is expected to more than quadruple within the next five years – from 4.9GW in 2018 to 22.2GW in 2023. Grid energy storage and electric vehicles will make up a large chunk of this.
But as energy storage demand increases, so do the amount of batteries that end up in landfills. Only 5% of lithium-ion batteries are recycled, saysJodie Lutkenhaus from Texas A&M University, one of the researchers behind the work. This is because recycling batteries is more expensive than just manufacturing new ones.
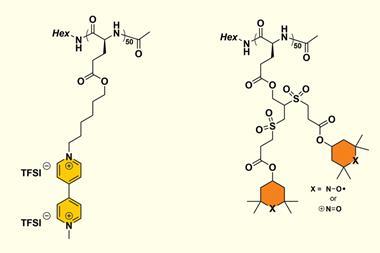
Lutkenhaus and her Texas A&M University colleagues Tan Nguyen and Karen Wooley have now built the first protein battery that is – in principle – fully biodegradable. In their 1.5-volt prototype, both electrodes are made from coils of polymerised glutamic acid units.
‘I envision that these batteries may first be used in single-use applications such as biometrics and health data monitoring,’ Lutkenhaus says.
To give the peptide its electron-shuttling ability, the team decorated the polymer chains with one of two redox-active compounds: (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, a stable radical better known as Tempo, on the cathode, and the bipyridine viologen on the anode.
Since the electrodes only contain peptides mixed with carbon particles, they could degrade by natural means – though in the current design the toxic viologen might pose a problem. Alternatively, ‘the battery peptides could be triggered externally to degrade into their original starting materials for reconstruction into fresh battery peptides,’ explains Lutkenhaus.
The proof-of-concept delivers up to 1.5V and 40–50 mAh/g for 50 cycles. After that, the battery loses capacity, possibly because the protein starts to dissolve in the electrolyte, Nguyen explains. While the prototype can’t yet compete with lithium-ion batteries – it needs to be three to five times larger to store the same amount of energy – ‘we hope this inspires others to think differently about the end-of-life fate for materials in batteries’, says Lutkenhaus.
Since polypeptides can be tailored create different 3D structures, the team is now working to uncover which arrangements give the best electron transfer performance.










No comments yet