Researchers have developed an organic redox flow battery that uses polypeptides as anolyte and catholyte materials.1 The concept could help to overcome sustainability problems with existing redox flow battery systems.
As the world pushes towards greener sources of energy, redox flow batteries are emerging as large-scale energy storage systems. However, a current issue with many redox flow batteries is that they rely on vanadium, an expensive and environmentally damaging metal, to act as a charge carrier. Researchers are therefore exploring organic redox-active materials as greener alternatives.
Now, having previously developed redox-active polypeptides2 for solid-state batteries, US research groups led by Jodie Lutkenhaus and Karen Wooley at Texas A&M University, along with Susan Odom’s research group at the University of Kentucky, have created a redox flow battery that uses viologen and Tempo polypeptides to shuttle electrons.
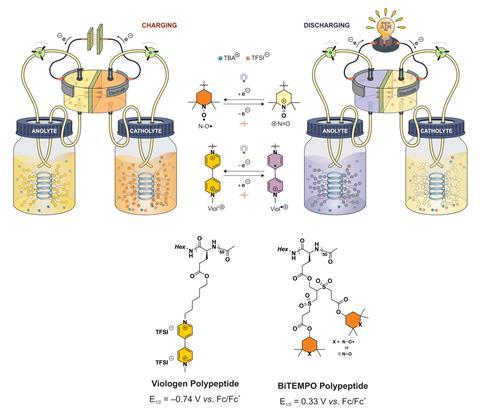
‘The polypeptide-based redox flow battery has shown good cyclability [500 cycles] and a very low capacity fade [0.1% per cycle],’ comments Shuang Gu, who develops redox flow batteries at Wichita State University in the US. But the most important aspect of the work for Gu is that the polypeptides are naturally occurring and biodegradable.
Ensuring the batteries can be recycled is a priority for Lutkenhaus, who says she aims to ‘close the loop’ on the matter. The team has already demonstrated that the polypeptides can be deconstructed simply by adding acid to them, which breaks them up into the individual amino acids. ‘The big challenge is once you have done the deconstruction you then have all these small molecules. How do you separate them out and reconstruct them back into the polypeptides again? In the future, we want to redesign this type of molecule so that the separation is very straightforward and easy and degrades into byproducts that we can just reconstruct without too many steps.’
Gu adds that making batteries from the most earth-abundant elements is a big advantage, along with ‘the anticipated low cost of manufacturing them’. But, ‘I would like to stress that [their cost] requires scrutiny and careful investigation. The cost of manufacturing most of the published organic redox materials does not seem to be much lower than making common inorganic redox materials.’ Lutkenhaus is also weary of cost and keeps it as one of her priorities, explaining that her aim is to design a battery made from materials that anyone can access. ‘Imagine if you could make a battery out of materials that you could source naturally from plants, or at the grocery store, then that means any global economy could make batteries,’ she adds.
This research study was dedicated to Susan Odom, who sadly passed away in 2021.
References
1 Z Liang et al, Mater. Adv., 2022, 3, 6558 (DOI: 10.1039/d2ma00498d)
2 T P Nguyen et al, Nature, 2021, 593, 61 (DOI: 10.1038/s41586-021-03399-1)









No comments yet