Reaction adds to research blurring the lines between transition metal and main group compounds
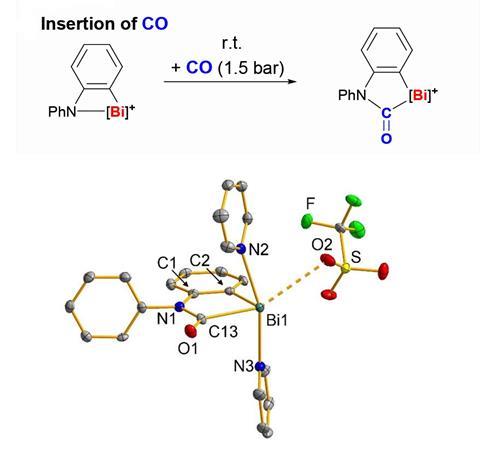
Scientists have replicated the reactivity of phosphorus–boron frustrated Lewis pair systems by inserting carbon monoxide into a Bi–N bond for the first time.
Carbon monoxide is a versatile C1 building block and as such is an important substrate for synthetic chemistry. However, the lack of synthetically accessible species based on heavy p-block elements containing orbitals of sufficient symmetry and energy for π-back donation into the metal—CO bond has rendered carbon monoxide a challenging substrate for insertion chemistry.
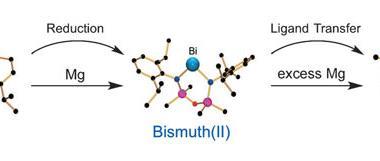
Now, researchers from Germany and the Netherlands have synthesised a dinuclear cationic bismuth amide with considerable ring strain leading to an elongated Bi–N bond. This predisposes the compound to high activity for small molecule activation. Indeed reacting it with carbon monoxide under mild conditions sees carbon monoxide inserted into the Bi–N bond, giving access to the first cationic bismuth carbamoyl species. The compound’s Lewis acidity and the release of ring strain drive the reaction.
This work reveals the potential of heavy main group compounds for small molecule activation, reversible electron transfer and photochemistry. In the future, organobismuth compounds may offer up further unexpected chemical reactivity pathways, blurring the traditional distinction between transition metal and main group compounds.








No comments yet