Click chemistry has been used to create antibody-based molecules that can treat cancer. These sorts of molecules are already in trials and have shown good anti-tumour activity in human cancer cell lines.
These checkpoint inhibitory T cell engager (CiTE) molecules can engage the body’s immune system and direct cytotoxic T cells to destroy cancer cells. They include a type of bispecific antibody known as a bispecific T cell engager (BiTE) that recruits the killer T cells and a protein that helps to suppress the immune dampening seen in cancer patients to make the treatment more effective.
Bispecific, or multi-specific, antibodies are a relatively recent concept in oncology and are designed to overcome some of the issues of older cancer drugs such as toxicity and drug resistance. To date, at least five bispecific antibodies have been approved and more than 200 are in development.
For the last two decades, protein engineering rather than chemistry has produced these biologic therapies. ‘If you go into any biotech company in this area, they’ll have a protein biochemistry and protein engineering group developing these molecules,’ says Ben Thomas, senior vice president, external innovation and operations at Oxford BioTherapeutics, a company specialising in this area.
Click chemistry, a simple method of joining two molecules together, saw Barry Sharpless, Morten Meldal and Carolyn Bertozzi awarded the Nobel prize in chemistry last year. In particular, Bertozzi was recognised for creating a copper-free biorthogonal version of the technique that can be used in living cells. While this click chemistry technology for creating CiTEs is still at an early stage, researchers are excited by its flexibility to adapt each candidate therapy by taking advantage of click chemistry’s Lego-like modular chemistry.
Many tumours are covered in sialic acids, which the cancer uses to help it evade the immune system. Bertozzi and team previously realised they could use a sialidase enzyme to strip cancer cells of sialic acids and make them more vulnerable to attack.
In 2019, Bertozzi gave a talk about this work at a chemical biology conference in London and spoke with fellow speaker Vijay Chudasama, a professor and group leader at UCL, while there. ‘We decided to reach out to her and see if we could combine what she was doing with the sialidase enzyme … with these bispecific T cell engagers,’ explains first author Peter Szijj, a postdoctoral researcher in Chudasama’s group.
The team created three-protein conjugates with two CiTEs, one with a common cancer checkpoint inhibitor and one with sialidase. ‘It’s very modular, because it’s really like Lego, you can take all these different proteins, modify them, and then stick them together in any order,’ said Szijj. ‘Whereas for protein engineering, they often have to do bespoke engineering for each different construct.’
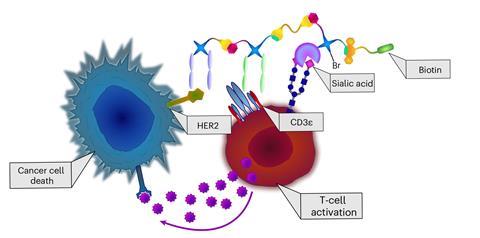
They found that the CiTE molecules were more effective than BiTE molecules for pushing the T cells to target cancer cells when they tested them in a cell model, particularly when they used sialidase as part of the molecule.
Szijj and colleagues now need to test their model further and examine scaling-up production before it can move into clinical trials. ‘There are maybe just two examples of this that are out there,’ says Chudasama. ‘We’re competing against something that’s been optimised for 20 years.’
‘I’m still not sure it will supplant the biochemistry-based approach,’ says Thomas. ‘It might do. But that’s where the evidence needs to be generated. Comparing the same molecules generated by the protein biochemistry compared to this chemical linkage would be interesting.’
References
P Szijj et al, Nat. Chem., 2023, DOI: 10.1038/s41557-023-01280-4














No comments yet