DNA–insulin nanoclusters have been created that can deliver a tuneable insulin signalling response in cells. The team from Sweden and Italy showed that the spatial organisation of insulin molecules on DNA nanorods can be used to control insulin–receptor activity. They propose that this technique could be used to develop targeted and more efficient treatments for diabetes.
The goal of insulin therapy is to mimic healthy blood sugar regulation in cells with insulin-resistant receptors. Ana Teixeira, a nanomedicine researcher at the Karolinska Institute, says that their ultimate aim is to develop a treatment that does not depend on a higher dose of insulin to produce a normal receptor response. ‘Can we make new variants of insulin that more effectively take advantage of the preserved activity that these receptors still have?’
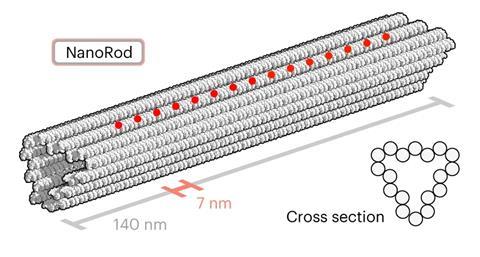
Insulin receptors can form clusters on cell membranes. In other biological contexts, interactions are stronger when a cluster of receptors interacts simultaneously with a cluster of ligands. The team investigated whether this would be the case for insulin–receptor interactions. The researchers chose DNA to build structures with as its base pairing made it an attractive material to create scaffolds for insulin. A folded ‘origami’ DNA–insulin rod 140nm long was designed and between one and 15 insulin molecules were attached at regular intervals.
Keeping the total concentration of insulin constant, the researchers tested the effect of the number of molecules attached to the rod (the valency). In in vitro fat cells, the valency and spacing of the nanorods affected insulin receptor activity with those with a valency of seven most effective at activating insulin signalling. These rods also triggered the transcription of genes associated with insulin signalling at a much lower concentration than unmodified insulin. To investigate shape effects in these clusters, nanorods were synthesised with seven insulin molecules with reduced spacing being the molecules. Moving the insulin closer together decreased receptor activation levels, showing that the geometry of the clusters, as well as the valency, controls the activity.
Teixeira says that the gene transcription results indicate that the nanorods are associated with longer-lasting receptor activation compared with unmodified insulin. ‘It seems to be a variable that is likely going to be different with nanocluster insulin, that the stability of the signal will be longer.’
The researchers also tested the nanorods in a zebrafish embryo model of type 1 diabetes, in which most of the cells that produce insulin were destroyed. They found that treatment with multivalent insulin nanorods stimulated glucose uptake – the desired response. By contrast, treatment with nanorods with only a single insulin molecule did not elicit a response.
Kirill Afonin, a researcher in RNA nanobiology at the University of North Carolina at Charlotte in the US, says that the work ‘not only shows promise for diabetes treatments but shows the potential for broader biomedical applications in addressing other diseases’. He adds that the system now needs to be studied in ‘clinically relevant models’. As a next step, the researchers intend to test the nanorods in mice.
Teixeira says they also plan to study the insulin receptor clusters in different types of cell, to develop nanocluster therapies that target individual tissues and eliminate unwanted receptor interactions.
References
J Spratt et al, Nat. Nanotechnol., 2023, DOI: 10.1038/s41565-023-01507-y











No comments yet