After more than 60 years, crystals of the 2-norbornyl cation have finally put its ‘nonclassical’ structure, with a pentacoordinate carbon, beyond doubt
German scientists have solved the long-sought crystal structure of an ion at the frontline of a war of interpretation that raged for years. Karsten Meyer from Friedrich-Alexander-University in Erlangen and his teammates have ended a 64 year wait for this most definitive evidence of ‘nonclassical’ 2-norbornyl carbocation structures. ‘The crystallography was a tour-de-force,’ Meyer tells Chemistry World. ‘The procedure is insane – it put us through torture.’
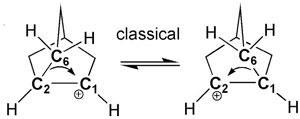
The 2-norbornyl carbocation became the focal point in conflicts over nonclassical pentacoordinate carbocations. In 1949, Saul Winstein proposed them to explain the reactivity of substituted norbornane compounds. But the suggestion that carbon might be bonded to more than four other atoms set warning sirens blaring. The leading opponent was Herbert Brown, who proposed a rapid equilibrium between two ‘classical’ tri-coordinated norbornyl cation enantiomers.
In the early 1960s George Olah, now at University of Southern California in Los Angeles, walked into the firing line. Both Winstein and Brown were interested in his carbocation work, which would win the 1994 Nobel Prize in Chemistry. ‘I discovered that carbocations, fleeting intermediates with very short lifetimes in acid catalysed processes including solvolysis reactions, could be stabilised in superacids and observed by spectroscopic techniques including NMR,’ Olah explains. ‘I disclosed this at the 1962 Brookhaven Mechanism Conference, where I met both Winstein and Brown. Each of them encouraged me to prepare and study 2-norbornyl cations under superacidic conditions to support their points of view.’
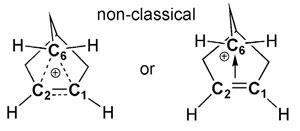
Olah got materials for those studies via Paul von Ragué Schleyer, now at the University of Georgia. ‘This was my PhD problem 60 years ago,’ Schleyer says. With their coworkers, Schleyer and Olah first saw 2-norbornyl cations by proton NMR in 1964. At room-temperature, fast hydride shifts and rearrangements meant the spectrum had only a single peak, but improved, lower temperature studies supported the nonclassical structure. Schleyer also reported the first adequately high level molecular orbital calculations that ‘gave the right answer for the right reasons’ and favoured the nonclassical structure.
Alongside ingenious experiments by many other groups through the 1970s and 1980s, their work convinced most chemists, if not Brown, of the nonclassical 2-norbornyl carbocation structure, Schleyer says. ‘But closure was not achieved until this modern X-ray crystal structure put a big rubber stamp on the whole file,’ he stresses. ‘After the first preparation of the stable ion in 1964, at least six research groups attempted, but failed, to solve the X-ray structure.’
Ideas crystallise: case cracked?
The ink for that stamp came from Ingo Krossing’s carbocation chemistry work at the University of Freiburg. His team uses soft bromoaluminate anions like [Al2 Br7]- to stabilise carbocations in the solid state, allowing Franziska Scholz to prepare appealingly white, regular, 2-norbornyl cation salt crystals. But when Krossing met with Meyer in February 2012 these crystals still refused to reveal their structural secrets to X-ray diffraction.
‘The crystals were particularly air and temperature sensitive, so they had to handle them at very low temperatures, and mount them in the diffractometer in 1-2 seconds,’ Meyer said. ‘Then they couldn’t obtain the fully ordered structure.’ Meyer wondered if, due to hydride shifts and rearrangements, the crystal might undergo a phase transition near the 100K temperature liquid nitrogen-cooled diffractometers can achieve. His team had recently purchased a helium cooled diffractometer, and so he proposed trying even lower temperatures.
But when his crystallographer colleague Frank Heinemann rapidly cooled a crystal towards 50K, it cracked. ‘The crystal undergoes an ordering process with severe lattice change through this phase transition,’ Meyer said. ‘The unit cell triples, which puts physical stress on the crystal. You have to anneal the structure by slowly warming up and cooling five, six, or seven times. At 40K, after expending a small fortune worth of helium, we finally got the fully ordered pattern of the norbornyl cation.’
During these efforts, Meyer and Schleyer met by chance at the computer chemistry centre that Schleyer founded when working in Erlangen. Knowing Schleyer’s history, Meyer mentioned the crystal structure progress. ‘I was going to a meeting, and when I got back to my office, I had half a dozen emails from him,’ Meyer said. ‘He gave so much input we made him co-author.’
Olah welcomes the new direct evidence for the nonclassical structure. ‘This impressive study fully confirms what we knew for a long time,’ he says. John D. Roberts at California Institute of Technology in Los Angeles, who first used the term ‘nonclassical ion’, calls the crystal structure discovery ‘excellent’ and ‘a lot of work’. But he adds that it still wouldn’t have changed Brown’s position. ‘Herb would be Herb no matter what happened,’ Roberts says. Schleyer agrees. ‘He might say, “Oh, but that’s solid state. I’m talking about solvolysis,”’ he suggests. ‘His arguments on solvolysis data interpretation were good, and many still have not been answered.’
References
F Scholz et al, Science, 2013, DOI: 10.1126/science.1238849







No comments yet