First examples of compounds in which a sulfide, chloride or bromide ion holds so large a coordination number
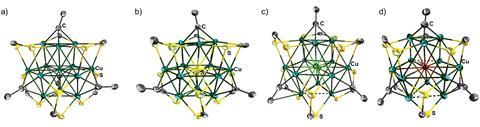
A team of scientists from Taiwan and France has synthesised a series of inverse-coordination clusters from a new copper-centred cuboctahedral arrangement. The precursor is the first copper-centred cluster to show cuboctahedral geometry, and can be further reacted to give sulfur, bromine or chlorine inverse-coordination clusters with the highest coordination number ever observed.
Inverse-coordination clusters are complexes with a non-metal at the centre surrounded by a number of metal atoms. High-coordinated molecules have previously accommodated a coordination-number of eight for chloride and nine for sulfur or bromide. The new inverse-coordination clusters see these ions reach a coordination number of 12.
Density functional theory calculations show that the covalent character of the bonds between the cage and the central atom decreases when the copper centre is replaced by a halogen. The chalcogen- and halogen-centred clusters also show enhanced luminescent properties.
The researchers encountered difficulties in preparing and separating these new clusters using spontaneous self-assembly so they devised a method that involved replacing a terminal alkynyl group with chloride.
References
This article is open access
K K Chakrahari et al, Chem. Sci., 2018, DOI: 10.1039/c8sc01508b









No comments yet