A mild and scalable electrolysis of carboxylic acids can provide streamlined access to valuable alkene feedstocks at kilogram scale. Using rapidly alternating polarity (rAP), the team in the US were able to boost the reaction performance and simultaneously expand the scope of these traditionally harsh reaction conditions to include pharmaceutically-relevant functional groups.
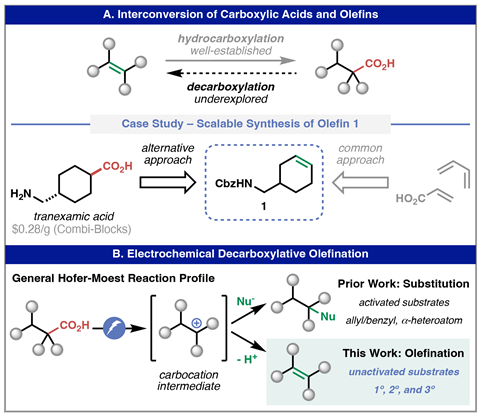
Both carboxylic acids and olefins are important chemical feedstocks and a reliable method to interconvert them would be a valuable addition to the synthetic toolkit. Hydrocarboxylation reactions – converting an alkene to the corresponding acid – are well-established but the limited repertoire of efficient and practical oxidation conditions make the reverse transformation far more challenging. Electrolysis is one of the few oxidative strategies that can transform carboxylic acids into their corresponding reactive intermediates – the reaction liberates carbon dioxide to produce either a radical or carbocation and variants giving different products have been known for more than 150 years. The Kolbe electrolysis, for instance, forms a hydrocarbon dimer via a radical mechanism while the Hofer–Moest reaction proceeds via a carbocation to form an alcohol. In each case, the reaction conditions are harsh and the reactive intermediates require a stabilising activated or tertiary system, limiting both the scope and product profile of these electrochemical reactions.
However, Phil Baran and his team at the Scripps Institute, US have now harnessed this electrochemical oxidation mechanism under milder conditions to efficiently convert a broader range of carboxylic acids into alkene products. The team began with a modified electrochemical decarboxylation setup, using graphite electrodes and a base to favour their desired reaction pathway. ‘The base is partially neutralising the carboxylic acid to generate a carboxylate anion, which is the suitable form to be electrochemically oxidised,’ explains Yu Kawamata, who co-led the project. ‘Then the carboxylate is oxidised on the anode surface, generating a carbocation intermediate which immediately loses a proton to form an olefin as the final product. A sacrificial protecting agent is also added to avoid over-oxidation of the olefin.’
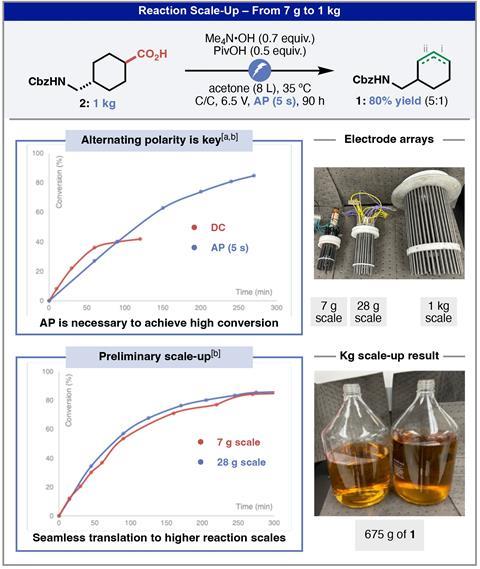
Importantly, the team applied an alternating polarity in this setup. Electrolysis typically uses DC current but, surprisingly, this completely suppressed the decarboxylation reaction. Analysis through a series of mechanistic and cyclic voltammetry studies revealed that DC current causes a localised buildup of electrogenerated acid around the anode, hindering deprotonation of the carboxylic acid and meaning only activated substrates can produce a measurable quantity of decarboxylation product. ‘By contrast, the alternating polarity cancels local acid accumulation by flipping the electrode polarity,’ explains Kawamata. ‘As a result, smooth decarboxylation sustains under these conditions, rendering the reaction much more general.’
With an optimised system established, the team were keen to investigate the performance of this reaction at an industrially-relevant scale. In partnership with process chemists at AbbVie, they adapted their lab-scale setup to a continuous stirred tank reactor, trialling the reaction first at 7g, then 28g and finally at 1kg. Each time, the reaction scaled smoothly and predictably and Baran and Kawamata are now working on optimising the process further for industrial applications and expanding the scope to tolerate more-challenging electron-rich functional groups.
Timothy Noël, a chemical engineer at the University of Amsterdam in the Netherlands, was impressed by the team’s results. ‘I really like this concept of rAP which allows one to reach higher reaction selectivity and greater functional group tolerance than you normally expect. Another strong feature is the seamless scale up from laboratory to kilogram range,’ he says. ‘There are some limitations to the scope but essentially the scale up shows the value and robustness of the chemistry. This method will find its way both in academia and industry.’
References
AF Garrido-Castro et al, Angew. Chem., Int. Ed., 2023, DOI: 10.1002/anie.202309157










No comments yet