Nearly 200 years since its discovery, industry rarely uses the carbon–carbon bond-forming Kolbe reaction – but now US researchers have shown it can sustainably make valuable substances.
Phil Baran’s team at Scripps Research Institute in La Jolla has done away with high voltages and platinum electrodes best established in the Kolbe reaction. In doing so, the researchers have made it much more versatile. ‘The most important feature is the ability to take waste or similarly priced products convert them into extremely high value materials,’ Baran tells Chemistry World.
Discovered by electromagnetism and electrochemistry pioneer Michael Faraday in 1834, and developed by Hermann Kolbe 15 years later, the reaction couples two molecules of a substance containing a carboxylic acid. Using a high direct current and a platinum electrode, the traditional reaction oxidises the starting material. The two molecules decarboxylate, emitting carbon dioxide and producing radicals that dimerise to form a new carbon–carbon bond. However, the harsh conditions usually oxidise other functional groups too, destroying the intended product.
The researchers hadn’t originally intended to explore the Kolbe reaction, explains Yu Kawamata, who co-led the project. Instead they were studying decarboxylation using the rapid alternating polarity (rAP) electrochemical approach they had previously developed and used for precise reduction reactions. They unexpectedly found that this led to Kolbe couplings, without unselectively oxidising other functional groups.
Kawamata explains that researchers have previously studied the Kolbe reaction using alternating current (AC) but had seen little difference from the original approach. ‘The key difference in this study is that we applied the rAP waveform to carbon-based electrodes rather than platinum electrode due to the need for practicality,’ he says. ‘This turned out to be a breakthrough for this project.’
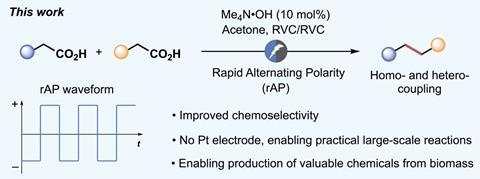
rAP avoids oxidising functional groups like double bonds, alcohols and ketones, allowing the chemists to synthesise potential monomer molecules that could polymerise to make more sustainable plastics. To demonstrate this, the Scripps team started from 10-undecenoic acid, which can be readily obtained from castor oil for about $20 (£16) per kilogram. The rAP-Kolbe reaction dimerises it to produce 1,19-eicosadiene, which currently costs $10,000 per gram. The researchers derivatised this to produce a diol, which they combined with a diketone to make a new bio-derived and degradable ketal polymer.
Another new chemical possibility is converting side-chain carboxylic acids of aspartic and glutamic acid to unnatural amino acids. ‘rAP-Kolbe can also provide a new, sustainable route to existing products,’ comments Baran. He highlights conversion of bio-derived succinic acid ester directly into adipic acid esters, currently mass-produced from petroleum-based chemicals.
Christo Sevov from Ohio State University in Columbus, US, says these results ‘represent some of the most significant advances to a reaction that has largely been plagued by the same limitations since its discovery’. ‘From our own experience, even substrates with functionality as simple as a double bond can completely inhibit decarboxylation by passivating electrode surfaces,’ says Sevov. ‘This work’s solution is the clever use of rapid alternating polarity that constantly switches the electrode surface from highly oxidizing to highly reducing and back.’
References
Y Hioki et al, Science, 2023, DOI: 10.1126/science.adf4762














No comments yet