US chemists have developed an electrochemical approach that precisely reduces single specific carbonyl bonds in molecules containing several. Rather than applying direct current (DC) as is usual in electrochemistry, Phil Baran’s team at Scripps Research Institute in La Jolla use rapidly alternating polarity (rAP). With this they selectively reduce just one carbonyl in various complex imide compounds.
‘This work is the first example of how a different waveform for delivery of electrical current can play a key role in defining product distribution within the context of complex organic molecules,’ Baran tells Chemistry World. ‘While pulsed techniques and AC current have been used to study reactions of simple compounds such as carbon dioxide, those studies have been ignored by the synthetic organic community.’
The discovery emerged after Baran’s team asked why electrolysis is always done using DC, explains team leader Yu Kawamata. Chemists hadn’t clearly answered whether alternating current (AC) would offer anything unique, he added. That was interesting because applying electricity to organic molecules can provide great control over reduction and oxidation processes. But DC-based electrolysis produces both reduction through one electrode and oxidation through another. Chemists usually only want one of these processes.
The Scripps researchers developed rAP to change the direction of electricity flow on the millisecond timescale, rather than 60 times a second like standard AC. The idea was to only harness fast electron transfer processes, avoiding slower ones. The chemists teamed up with lab equipment producer IKA, which implemented an rAP function on the commercially available ElectroSyn2.0 potentiostat at its San Diego site.
Targeting the most reactive site
To test the approach, the Scripps team studied a phthalimide molecule containing activated carbon–hydrogen bonds and two imide carbonyl groups, which could potentially be oxidised and reduced, respectively. In conditions containing electrolyte reagents, rAP only reduced one carbonyl group, without oxidation. Increasing current pushed the reaction beyond reducing the carbonyl to an alcohol group, removing the alcohol group and leaving just a carbon–hydrogen bond. DC electrolysis gave complex mixtures of oxidation and reduction.
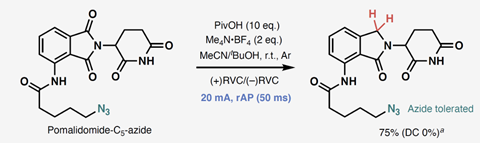
Exploring different molecules, the approach selectively reduced phthalimide carbonyls in the presence of reducible alkene, alkyne, epoxide, alkyl bromide and ketone groups. rAP also reduced a phthalimide carbonyl in pomalidomide, an important component of potential proteolysis-targeting chimera (Protac) drugs, while it was attached to an azide linker.
Electrochemistry’s selectivity originates from single-electron transfer (SET), explains Kawamata. ‘This is where redox potentials or frontier molecular orbital theory come in,’ he says. ‘What they tell you is basically which carbonyl groups have the greatest tendency of accepting electrons, and rAP is particularly good at targeting the most reactive site.’ ‘This points to a completely new optimisation variable, exclusive to electrochemistry, that enables precise control of chemoselectivity, one of the most important challenges of organic chemistry,’ adds Baran.
Long Luo, an electrochemist from Wayne State University in Detroit, US, calls the study ‘truly exciting work’. ‘It clearly demonstrates the unique chemical reactivity that AC electrolysis can offer for controlling reaction pathways,’ Luo says. ‘The strength of this work is that they have explored an enormous substrate scope and demonstrated its tremendous potential for applications in the synthesis of pharmaceutical compounds.’ However, currently finding optimal reaction conditions relies heavily on trial-and-error and lacks rational design, which might limit the wide application of this method, Luo adds.
The Scripps team is now exploring redox reactions of various functional groups under rAP conditions. ‘Since historically almost all electrochemical reactions have been studied under direct current, rAP opens a new territory where barely anything is known,’ Kawamata says. ‘We are very excited to see the new discoveries awaiting in this area.’












No comments yet