Zinc can have a +3 oxidation state, new theoretical research shows. The work challenges a fundamental concept of chemistry by showing that zinc’s core electrons can take part in chemical bonding.
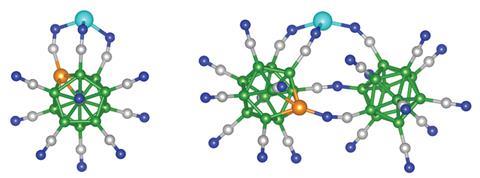
Until recently, zinc was thought to be limited to the +2 oxidation state due to its electron configuration being [Ar] 3d10 4s2. Now, scientists from the Virginia Commonwealth University, US, have calculated that if zinc interacts with highly stable super-electrophilic trianions it can create compounds with zinc in a +3 oxidation state.
Previous attempts to coax zinc into a +3 oxidation state explored using three separate anions but failed as the anions bonded to each other forming dimers, instead of bonding to zinc. Here the researchers have used single trianions, BeBu11(CN)123- or BeB23(CN)223-, to avoid such complications. The trianions can gain three electrons from zinc whilst remaining highly stable and mean that zinc’s core d electrons would participate in zinc–nitrogen bonds.
By exhibiting variable oxidation states, zinc’s behaviour aligns more closely with other transition metals.
References
H Fang et al, Nanoscale, 2021, 13, 14041 (DOI: 10.1039/d1nr02816b)








No comments yet