Researchers have created a set of polymers that cannot be made via solution-phase reactions, using a ball-milling method.1
Ring-opening metathesis polymerisation is a synthetic technique for producing functional polymers. It’s typically conducted in a liquid, which limits reactions to those where the monomers and initiator share a common solvent. As a result, many polymers remain unexplored or require multi-step processes or post-polymerisation modifications.
Mechanochemical polymerisations were first reported in the 1950’s and are of increasing interest to the green chemistry community as they use little to no solvent. But compared to examples of solution-phase ring-opening polymerisations, methods for mechanochemical ring-opening polymerisation are limited.
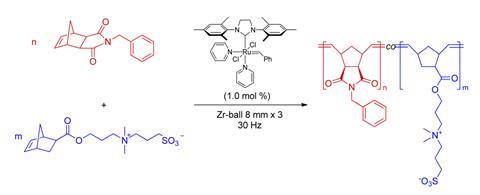
Noting this synthetic gap and given recent work using ruthenium-alkylidene catalysts for ring-closing and cross-metathesis reactions under ball-milling conditions,2 a team based in South Korea and Canada has performed ring-opening metathesis polymerisation using a similar set-up. They explored the polymerisation of immiscible monomers, theorising that mechanochemical methods would remove the need for a common solvent.
The investigation explored a variety of solid monomers including ionomers, a fluorous monomer and macromonomers to determine the scope and functional group compatibility of the initiator. While ring-opening metathesis polymerisation using ionic monomers was previously reported in aqueous media, the team had to modify the initiator to make it water soluble. While they initially obtained lower conversions, liquid-assisted grinding using water or DMF improved the efficiency of the reactions.
Notably, the team achieved high conversions for copolymerisations of monomers of orthogonal solubility, regardless of the polarity of the monomers. This involved liquid-assisted grinding with THF and water, with reduced solvent usage compared to solution-phase methods.
References
1 G S Lee et al, Chem. Sci., 2022, DOI: 10.1039/d2sc02536a (This article is open access.)
2 J-L Do et al, J. Am. Chem. Soc., 2015, 137, 2476 (DOI: 10.1021/jacs.5b00151)










No comments yet