Scientists in Canada have united mechanochemistry with repurposed lab equipment to develop a new synthesis and crystallisation process. It is scalable and efficient, uses little to no solvent and combines the crystallisation and drying processes, so could benefit the pharmaceutical industry by saving it time and money when making active pharmaceutical ingredients, as well as being less damaging to the environment than current methods.
Traditional organic synthesis and crystallisation processes are relatively solvent intensive. However, solvent use and recovery is associated with significant energy use and greenhouse gas emissions as well as vast volumes of waste solvent.

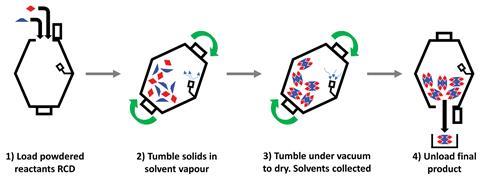
To eliminate this waste problem and improve the environmental credentials of these processes, Alex Stirk, from Apotex Pharmachem in Ontario, and his team have developed a process they call vapour assisted tumbling. ‘If all you are using your solvent for is to get good collisions, then if you agitate your reactants in some kind of device, in this case a rotary cone dryer, you can drastically lower the amount of solvent you actually use,’ explains Stirk.
Rotary cone dryers are commonly used in industrial chemistry settings to dry solids. Stirk’s team has shown that small modifications can covert them into reaction vessels that can tumble two solids together in an atmosphere of solvent vapour to form the desired product. This mechanochemical technique can also work on a smaller scale in an academic environment using a standard rotary evaporator. In both cases, the equipment is already designed to trap the solvents being removed from drying solids, so as well as needing a much smaller amount of the solvent, it is possible to recover and recycle it. This greener crystallisation process saves both time and money.
Tomislav Friščić, a green chemist at McGill University in Canada, who was not involved in the research, says ‘people have done such things before as a concept, but the beauty of this work is that it shows a very simple way that low energy, low solvent chemistry can be implemented in an industrial setting.’
The team has already shown this process can be used to produce several pharmaceutically active solid forms, including salts and cocrystals of well-known drugs such as acalabrutinib, olanzapine, aripiprazole and even caffeine. Stirk is optimistic about other potential applications of the technique. ‘If someone else wants to try this themselves, they won’t have to buy any new instrumentation at all, the functionality that they need is already there, there is just a different thought process, “can I use this process for my reaction?”’ says Stirk. ‘You are only really limited by your imagination, the sky’s the limit.’











No comments yet