Scientists have synthesised a new class of organometallic molecules – metal-centred planar annulene frameworks. These new compounds hold promise as building blocks in materials science, potentially finding use in electronics, catalysis and photonics.
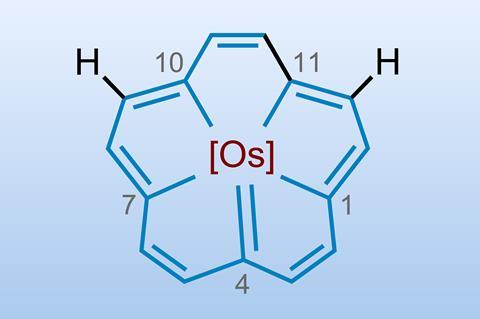
Annulenes are monocyclic hydrocarbons with the general formula CnHn or CnHn+1 when n is an even or odd number, respectively. The best known example is benzene. They can form coordinates with various metals to form metal complexes where the metal sits above or below the plane of the flat annulene anions – ferrocene is an example of an out-of-plane annulene complex with an iron atom sandwiched between two cyclopentadienyl rings. The synthesis of three metal-centred [15]planar annulene complexes is a first for the field. Incorporating a metal within the plane has been synthetically challenging due to issues such as ring flexibility and restrictions on annulenes’ cavity size.
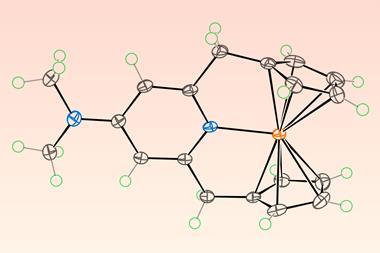
A team of researchers from China and the US overcame these obstacles through targeted molecular design to create a 15-carbon annulene – five fused rings bonded to osmium at the centre. The osmium forms a sigma bond with the carbon atoms and sits within the ring. The team employed a novel method of building the annulene framework around the metal centre, rather than inserting the metal into the system. Their four-step synthesis started with a precursor containing a reactive osmium–carbon triple bond and then involved assembling carbon–carbon bonds around the metal atom via cycloaddition reactions.
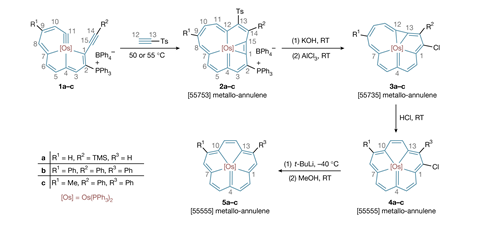
This route resulted in the synthesis of three in-plane metallo-annulenes and then converting the symmetrical, parent molecule to the corresponding chlorinated, iodinated and nitrated derivatives. Characterisation revealed that the structures exhibited characteristics similar to expanded porphyrins – highly conjugated planar molecules with a hollow centre where a metal atom can sit – but without heteroatoms. The osmium complex displayed high stability and functionality.
The work redefines metalla-aromaticity by bridging classic annulene chemistry and porphyrin coordination chemistry adding to fundamental bonding theory. Since the researchers’ strategy worked with osmium, they now plan to adapt it to other transition metals to create a diverse family of planar metal–carbon aromatic systems.
References
S Huang et al, Nature, 2025, 628, 520 (DOI: 10.1038/s41586-025-08841-2)













No comments yet