Simulations help scientists identify elusive mechanism generating atmospheric sulfuric acid
A theoretical study has unravelled the chemistry surrounding how sulfur dioxide and amines react in the atmosphere, leading to an explanation for the high levels of sulfuric acid in heavily polluted air.
Sulfate air pollution is partially responsible for the light-blocking hazes that regularly dog megacities like Beijing. Amongst other sources, coal-fired power plants and coal-burning factories emit sulfur dioxide, which becomes sulfuric acid when it is oxidised by hydroxyl radicals. However, there aren’t enough hydroxyl radicals in the atmosphere to account for the high levels of sulfuric acid recorded in these conditions.
Now, a team of researchers in the US and China, led by Joseph Francisco from the University of Pennsylvania, has used various computer simulations to uncover a previously unknown chemical mechanism that generates atmospheric sulfuric acid. ‘Amines, including dimethylamine, are found to play a key role in atmospheric nucleation,’ explains Francisco. Most atmospheric amines originate from biological processes, but vehicle emissions are another significant source.
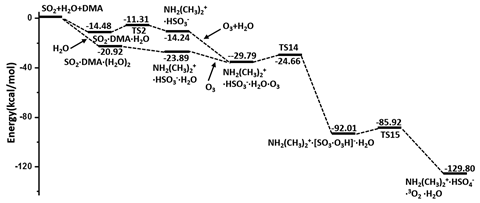
The team found that dimethylamine hydrates sulfur dioxide through exothermic hydrolysis, making sulfurous acid. This can either be oxidised by ozone or nitrogen oxides, both found in the atmosphere. Ozone is actually a stronger oxidant than nitrogen oxides here, so ozone then oxidises sulfurous acid to sulfuric acid.
‘What excites me is that the hydrolysis of sulfur dioxide with dimethylamine is a spontaneous process. The hydrolysis rate of sulfur dioxide and dimethylamine with water vapour becomes highly competitive with the rate of sulfur dioxide and the hydroxyl radicals, especially under the conditions of heavily polluted air at high humidity, while nitrogen oxides appear to play a much less significant role than ozone in the aqueous oxidation of sulfur dioxide,’ explains Francisco.
Christian George, an atmospheric chemist at the CNRS-Institute of Research on Catalysis and Environment in Lyon, France, says the study ‘describes a new chemical mechanism that may operate (or not) in the troposphere and may affect our understanding of how sulfate is produced’. ‘It shows again that the chemistry of sulfur dioxide, despite being investigated for decades, still contains significant unknowns, which is interesting and probably eye opening for certain colleagues.’












No comments yet