For the first time, researchers have measured the live formation of chiral bonds at the single-molecule level. The method could uncover ‘previously unknown reaction mechanisms of enantiospecific reactions, which is [currently] really hard and time consuming,’ explains materials chemist Sascha Feldmann from Harvard University, US, who wasn’t involved in the project.
The detection of chemical properties like chirality generally involves macroscopic measurements. ‘[This] brings ensemble-averaged results,’ says Xuefeng Guo, from Peking University, China. Now, Guo’s team has developed a way to monitor the formation of individual chiral centres in real time.
‘This is the first time that such a complex reaction was monitored in situ, in real time and at the single-molecule level,’ says Guo. He notes that the unprecedented level of detail provided by the technique allowed the team to characterise and ‘understand in full the different reaction steps’.
To do this, Guo’s team used graphene-based molecular junctions – a kind of microscopic electronic component. ‘[They could] be regarded as resistors,’ explains Guo. ‘The basic device architecture is an electrode–molecule–electrode sandwich structure.’
Any chemical changes to the integrated molecule cause changes to the junction’s electrical resistance, which are detected by continuous current measurements.
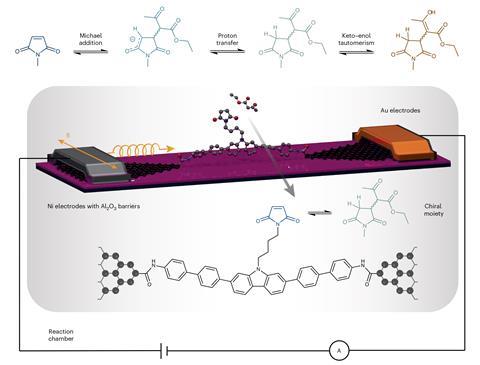
In Guo’s experiment, the ‘sandwiched’ molecule was functionalised with maleimide, a classic substrate in asymmetric Michael additions. When anions of 1,3-dicarbonyl compounds attack maleimide – the Michael acceptor – a chiral centre is formed. The experiment’s design means that the researchers can tell which enantiomer is formed.
The detection of different enantiomers is possible because of the chirality-induced spin-selectivity effect (Ciss). ‘Ciss was discovered … about two decades ago,’ explains Feldmann. ‘It’s based on the observation that a current of electrons becomes spin-polarised upon passing through a chiral medium.’ In the new work, this is used to detect the chirality of the reaction product and intermediates in situ, adds Feldmann.
‘Enantiomers [would] show the same resistance without spin injection,’ says Guo. ‘The adoption of the Ciss effect makes the chirality measurable.’
To determine the exact stereospecific structure of the reaction intermediates, the researchers prepared different molecular junctions as control experiments. By anchoring molecules with known chiral configurations to the single-molecule detection device, the measurement with spin-polarised current could identify chiral information, explains Guo. ‘Ultimately, these assignments were corroborated by additional measurements, [including] inelastic electron tunnelling spectra and theoretical calculations,’ explains Feldmann.
Additionally, the experiments provide powerful proof of the mechanism and origins of the Ciss effect, corroborating a recently reported theory. ‘The single-molecule junction architecture provides experimental support for the so-called “spinterface theory”, [and helps] better understand the mechanism of the Ciss effect,’ explains Feldmann. This idea suggests the effect appears in interface interactions between the electrode and the chiral molecules. Simulations based on the spinterface theory clearly coincide with the new experimental data, thus ‘[clarifying] the mechanism of the controversial Ciss effect, [and enabling us] to reversibly change the chirality of the chemical reaction in situ,’ says Guo.
According to the Feldmann, this final feature could one day be used to control the stereochemical outcome of reactions, without auxiliaries or chiral catalysts. ‘This active chirality-induction mode, [could] save a tremendous amount of resources and enable the synthesis of previously inaccessible products,’ he says.
In the nearer term, the technique could provide improved analysis of chirality changes during chemical reactions. Guo believes the technique could lead to the discovery of ‘a large amount of microscopic individual information, including the transmission and amplification mechanisms of chirality, and the evolution of asymmetric reactions at a molecular scale’. Feldmann notes that such findings could directly help the pharmaceutical industry manufacture drugs more efficiently.
References
C Yang et al. Nat. Chem. 2023, DOI: 10.1038/s41557-023-01212-2















1 Reader's comment