Combining synchrotron x-rays with scanning tunnelling microscopy gives atomic resolution
A new technique demonstrated by researchers in the US combines x-ray characterisation with scanning tunnelling microscopy to achieve both single-atom resolution and chemical specificity of atoms on surfaces. The synchrotron x-ray scanning tunnelling microscopy (SX-STM) technique even provides information about the atoms’ oxidation states.
Since its invention in 1981 by Gerd Binnig and Heinrich Rohrer at IBM Zurich in Switzerland, STM has become an indispensable tool for scientists studying surfaces with atomic resolution. It provides detailed topographic information by measuring the variations in current as electrons tunnel between a surface and an atomically sharp probe held close to the surface. It cannot, however, reveal much about its chemical identity, because only the valence electrons tunnel to the tip.
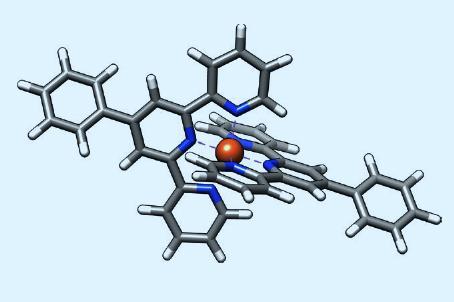
‘We need x-rays, because x-rays excite the core-level electrons which are the fingerprints of the atoms in the periodic table,’ explains Saw-Wai Hla at Ohio University and Argonne National Laboratory (ANL) in Illinois. However, nobody has yet managed to detect fewer than around 10,000 atoms using x-rays. The researchers decided, therefore, to combine the two techniques by using x-rays to excite core electrons to the valence bands of atoms, where they could be detected with atomic resolution by tunnelling to an STM tip.
Hla and colleagues used SX-STM to study ring-shaped supramolecular assemblies formed by terpyridine ligand subunits on a gold surface. The subunits in each assembly were linked by ruthenium ions, except for a single link containing an iron ion. They used traditional STM to keep the tip at a constant height as they scanned the sample, but removed the tunnelling current from the final measurement. They then bombarded the surface with pulses of monochromatic x-rays produced by ANL’s Advanced Photon Source. When the tip was positioned around 0.5nm over the iron on the surface, the researchers could detect electrons from just a single atom, producing the characteristic x-ray absorption spectrum of iron. They also studied another supramolecular complex containing terbium, and resolved two ions 1.24nm apart.
The researchers then fixed the tip over specific atoms and slowly increased the x-ray photon energy. By looking at the peaks at which the tunnelling current suddenly spiked, and comparing to known x-ray spectra, they showed that the iron was in the Fe(II) state, whereas the terbium was in the Tb(III) state. However, some peaks were missing from the spectra. Density functional theory calculations revealed that the orbitals giving rise to these peaks are predicted to lie in the plane of the surface, and thus unable to interact with the STM tip.
By measuring the exact energies of the peaks, the researchers also showed that, unlike iron, terbium does not hybridise with its neighbouring nitrogen atoms. ‘Terbium is isolated because the 4f electrons are isolated by the 5d and 6s electrons of the rare earth. That highlights why [rare earths] are extremely interesting, because if you change the number of 4f electrons you change the properties completely.’ The researchers now want to look at potential practical applications such as studying the origins of protein misfolding.
‘[These researchers] have been driving and edging towards this for the last 10 years or so and it’s come to fruition,’ says condensed matter physicist Philip Moriarty of the University of Nottingham in the UK; ‘There’s still unanswered questions, but the very best science is always unanswered questions and the fact that their signals are so large gives the rest of us hope in setting this up and trying it for ourselves … Is this going to be something that every university and every R&D lab has as a go-to technique? Probably not. However, it’s pretty much unprecedented, in that it combines core-level analysis – and all its advantages in terms of chemical specificity – with single-atom resolution. That is very much a leap forward.’
References
T M Ajayi et al, Nature, 2023, 618, 69 (DOI: 10.1038/s41586-023-06011-w)

















No comments yet