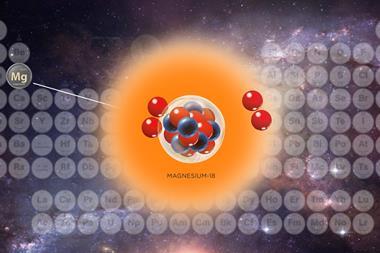
The lightest isotope of uranium to date has been created in China, and researchers believe it could help to understand the complex nuclear forces at play inside an atom as it decays.
Working at the Heavy Ion Research Facility in Lanzhou, China, an international team of collaborators led by Zhiyuan Zhang used a particle accelerator to fire argon ions into a tungsten target, hoping to create uranium isotopes with far fewer neutrons than usual. When they separated out the products of the reaction, the team discovered a previously unknown isotope, uranium-214, which has just 122 neutrons – 24 neutrons fewer than the most abundant uranium isotope, uranium-238.
There are 254 stable isotopes that exist in nature on Earth, but more than 3300 have been identified through experiments in particle accelerators. This has been either as the result of nuclear fusion from smashing particles together, or as ‘daughter’ isotopes of these unstable, radioactive creations break apart. Typically, this involves alpha decay, where the nucleus loses alpha particles (two protons and two neutrons), degrading into a lighter element.
By measuring uranium-214’s alpha decay chain, which saw the new atom break down into previously known isotopes of thorium, radium and radon, the team were able to pinpoint the half-life of uranium-214 at just 0.52ms. The team were also able to better characterise the decay chains of two other previously known uranium isotopes, uranium-216 and uranium-218.
But creating a new isotope was only part of the experiment. The half-lives of isotopes are affected by their numbers of protons and neutrons, with increased stability seen around so-called ‘magic numbers’, such as neutron number 126. However, the interaction between a nucleus’ protons and neutrons is less well understood, particularly for heavier elements beyond lead, and the researchers hoped to measure how this influenced alpha particle formation.
The team noticed that, as the uranium atoms decayed, the changes to the widths of uranium-214 and uranium-216 were twice that expected, suggesting a strong monopole interaction between the atom’s protons and neutrons that increased the probability of alpha decay. This observation could give future researchers a better idea of how a nucleus breaks apart and forms an alpha particle, furthering our understanding of nuclear structure.
Thomas Albrecht-Schönzart, a professor in actinide chemistry at Florida State University, US, describes the work as ‘pushing our understanding of nuclear stability farther than previously possible’. ‘The real achievement is the explanation for the stability of this very neutron-poor isotope via the strong monopole interaction between the proton and neutron spin-orbit partners,’ he says. ‘From a radiochemist’s perspective, the ability to precisely determine the half-lives of isotopes this short-lived is also remarkable.’
However, Witold Nazarewicz, professor of physics at Michigan State University, US, urges caution, pointing out that the team’s level of experimental uncertainty suggested more work was required. ‘A discovery of a new isotope is obviously a nice thing, but I do not believe they can say anything profound about proton–neutron interactions,’ he says.
References
ZY Zhang et al, Phys. Rev. Lett., 2021, 126, 152502 (DOI: 10.1103/PhysRevLett.126.152502)














1 Reader's comment