Macrocyclic NHC ligands serve up trio of new actinide sandwich complexes
Delocalised, anionic macrocyclic NHCs with unusually strongly σ-donation set to expand suite of stable organometallic complexes with f block cations
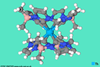
Researchers in the US have synthesised the first macrocyclic N-heterocylic carbene (NHC) complexes to incorporate elements from the f block and investigated their unusual electronic structures.