A new microscopy technique has given researchers greater insight into the way that droplets and bubbles form. This new understanding could enable researchers to design materials that control the nucleation of bubbles and droplets – a crucial aspect of many industrial processes.
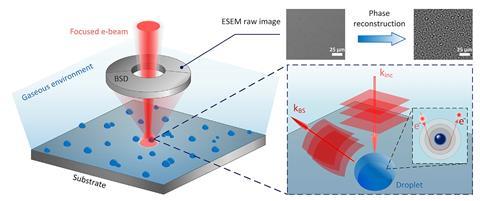
The formation of bubbles and droplets is at the heart of many chemical processes, from fundamental events like boiling and condensation to complex reactions like water splitting. Now a new scanning electron microscopy (SEM) technique has enabled researchers to observe the nucleation process in more detail than ever before.
The research team, led by Evelyn Wang from the Massachusetts Institute of Technology, US, used phase-enhanced environmental SEM to analyse nucleation site distribution during condensation, chemical vapour deposition and a hydrogen evolution reaction. With this technique Wang’s team could directly image electrons at a sub-micrometre scale, allowing them to build a detailed image of nucleation sites.
The group showed that the density with which nucleation sites are populated is governed by a Poisson distribution, as expected. However, the spatial distribution – the nearest-neighbour distance – followed a Rayleigh distribution, which was unexpected given that chemical engineers have assumed for decades that this was also governed by a Poisson distribution.
Understanding this microscopic phenomenon will give scientists greater insight into the macroscopic behaviour of many materials. The researchers say that the new SEM tool will help them understand the role of nucleation site distribution in phase-change heat transfer, gas-evolving reactions, and material growth.
References
L Zhang et al, Cell Rep. Phys. Sci., 2020, DOI: 10.1016/j.xcrp.2020.100262













No comments yet