Nanoparticles can now be made from metals that won’t normally mix. This advance allows the materials’ properties to be fine-tuned for a range of different applications such as improved catalysts.
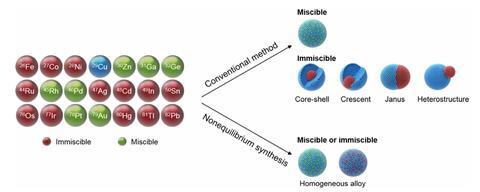
Bimetallic nanoparticles are important materials that combine different metals’ unique characteristics into new structures with enhanced chemical properties. But not all metals mix together easily.
This means that some combinations of metals that could, in theory, make interesting new catalysts are almost impossible to prepare. With conventional methods, these metals would tend to form layered structures or particles with localised areas of each metal – rather than a fully dispersed mixture.
Now, a team of US researchers has found a way to make homogenously mixed bimetallic nanoparticles, opening up a whole new range of new materials for investigation.
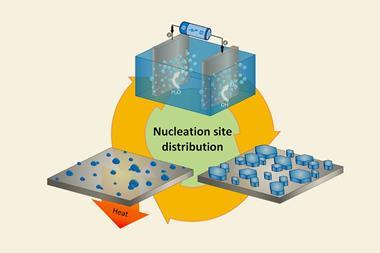
‘We have developed a unique method for mixing metals – regardless of their immiscibility at the nanoscale – to create a new range of bimetallic materials,’ says Chunpeng Yang, who worked on the project at the University of Maryland, College Park. The team first soak carbon nanofibres in solutions containing two different metal nitrates. Once these have dried, the researchers pass a rapid pulse of electric current through the nanofibres – heating them up to over 1300°C for less than a quarter of a second. This shock causes the nitrates to decompose, while the metals are trapped in nanoparticle form, without time to separate before they solidify.

The innovative strategy allows the group to overcome the immiscibility of the constituent elements, says Xue Wang, who designs electrocatalysts at the University of Toronto. ‘In this way they prepare a series of homogeneously alloyed copper-based bimetallic catalysts with a wide range of atomic percentages of copper,’ explains Wang, who wasn’t involved in the project. ‘This is difficult to do using previously-reported synthetic methods,’ she adds.
The technique was used to mix copper with six different metals: nickel, silver, indium, palladium, zinc and tin. The team also prepared copper–silver nanoparticles with varying proportions of either constituent. These particles were then tested out as catalysts for the electroreduction of carbon monoxide – a reaction that can produce a range of useful fuels and feedstocks.
‘We discovered that the incorporation of nickel or silver into copper nanoparticles suppresses the undesired hydrogen evolution reaction while promoting the production of multicarbon products,’ says the University of Delaware’s Feng Jiao, who also worked on the project. ‘In particular, Cu0.9Ni0.1 achieved an exceptionally high acetate formation with 46.7% Faradaic efficiency, which is among the highest reported to date for CO electroreduction.’
Yang suggests that the method could be used by scientists to create diverse nanoparticle systems with potential applications in catalysis, biology, optics and magnetic materials.
‘Almost any combination of multiple elements can be made into a single nanoparticle with a uniform elemental distribution,’ adds Jiao. ‘The next step is to further exploit multi-component systems, for example high entropy alloys, for potential electrocatalytic reactions, such as CO2 reduction and electrosynthesis.’
References
C Yang et al, Sci. Adv., 2020, DOI: 10.1126/sciadv.aaz6844














No comments yet