
Even though radioactive radium fluoride molecules exist for just a fraction of a second, scientists have made them and found that they could extend our fundamental understanding of physics. An international team of scientists used a particle accelerator at Cern in Geneva, Switzerland, to produce the molecules, and studied them spectroscopically before the radium atoms decayed.
Some physics theories suggest that the unique nuclear properties of radioactive radium isotopes the team studied could dramatically enhance the violation of symmetries in the standard model of physics. ‘This can change our fundamental understanding of nature with a molecular experiment, which I think is absolutely fantastic,’ comments team member Robert Berger, from Philipps-University Marburg, Germany. ‘I can take a molecule and learn something about how the universe is working.’
Berger is a theoretical chemist, and found with other scientists that radium fluoride spectroscopy might test the weak force’s symmetry about a decade ago. But no-one was able to do the experiments until he met Ronald Garcia Ruiz at Cern in 2017. ‘I was told it was impossible,’ recalls Garcia Ruiz, who now works at the Massachusetts Institute of Technology in US. ‘With these molecules nothing was known.’
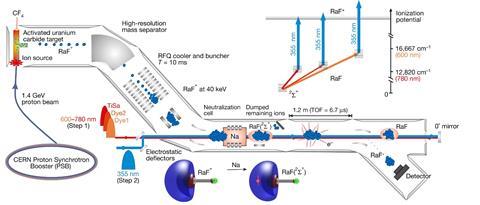
Garcia Ruiz and his collaborators studied radium fluoride using Collinear Resonance Ionisation Spectroscopy (Cris). His team fires protons at a uranium carbide target, activating it before mixing in carbon tetrafluoride molecules at high temperature and producing positively charged radium fluoride ions. ‘At the beginning we’re producing 106 atoms per second in a very hot and highly contaminated environment,’ Garcia Ruiz says. ‘You can only produce less than one nanogram. So you need to get this molecule out and study it with some precision.’
The team isolated radium fluoride ions by exploiting the same principles that separate ions in mass spectrometry. The ions then pass through a chamber filled with sodium gas, which removes their positive charge. Then laser pulses add energy, raising the radium fluoride’s electronic energy level, and ultimately ionising it again. The newly positively charged ion can then deflect into a detector. Tracking how many ions reach the detector at different laser wavelengths gives the scientists radium fluoride’s excitation spectrum. ‘You are using perhaps 10 molecules in the state that you want to study,’ explains Garcia Ruiz. ‘You really need the ability to study molecule by molecule.’
Producing and studying radium fluoride like this is an ‘important intermediate result’, Berger says. The findings suggest that they can now use laser cooling, where molecules absorb and re-emit photons and slow down, enabling higher precision spectroscopy. Scientists will be able to test their ideas about the limits of physics. ‘Collider centres were waiting for this,’ adds Berger.
Conducting such a high-precision study ‘is certainly a challenging experimental task’, says Emine Altuntas from the US National Institute of Standards and Technology and Joint Quantum Institute, who wasn’t involved with this work. She calls the results a ‘crucial and an exciting step’. ‘The method presented in this study is versatile as it can be applied to different isotopes, which will be a key factor in the investigation of the systematic effects,’ she says.
References
R F Garcia Ruiz et al, Nature, 2020, DOI: 10.1038/s41586-020-2299-4
















No comments yet