Chemists overcome synthetic challenges to make uncharged carbon molecules that sit at far end of THF basicity scale
Researchers have developed a new family of non-ionic carbon superbases featuring a carbodiphosphorane group.1 These air-stable structures are the most basic uncharged carbon bases to date.
Since Reinhard Schwesinger at the University of Freiburg in Germany reported in 1987 that phosphazenes could act as organo-superbases,2 chemists have sought to develop non-ionic organic bases that match the basicity of common inorganic or organometallic bases. Non-ionic bases usually require basic nitrogen groups such as imines (N=C) or phosphazenes (N=P), but three years ago Jörg Sundermeyer and his team at the University of Marburg reported that a phosphorous ylide (P=C) showed exceptional basicity.
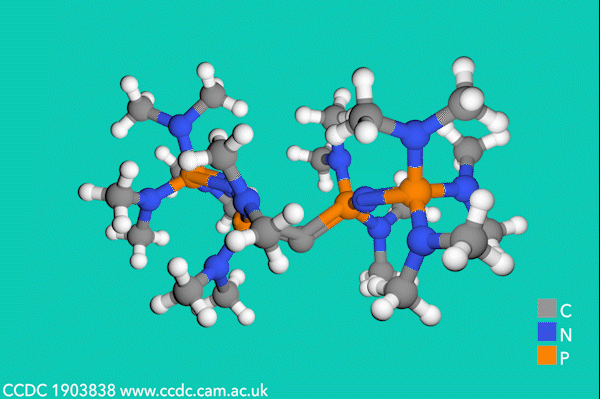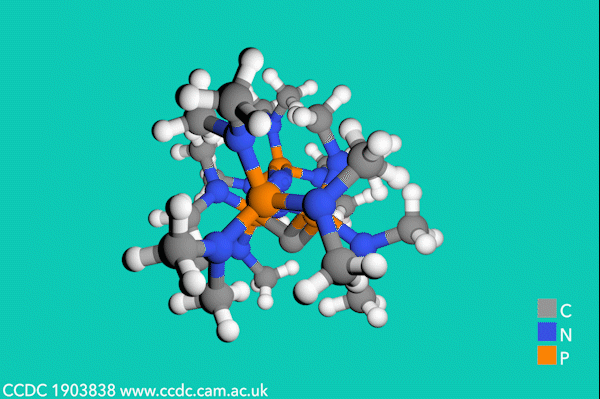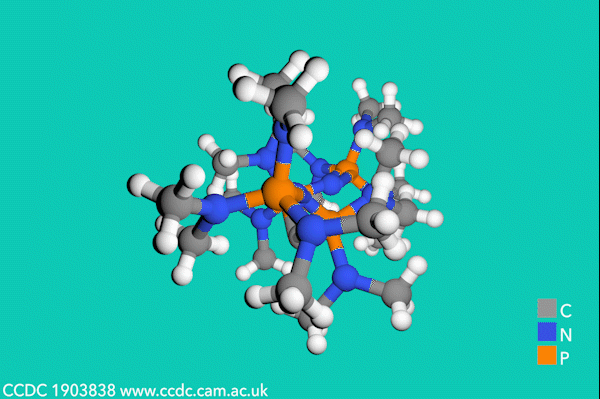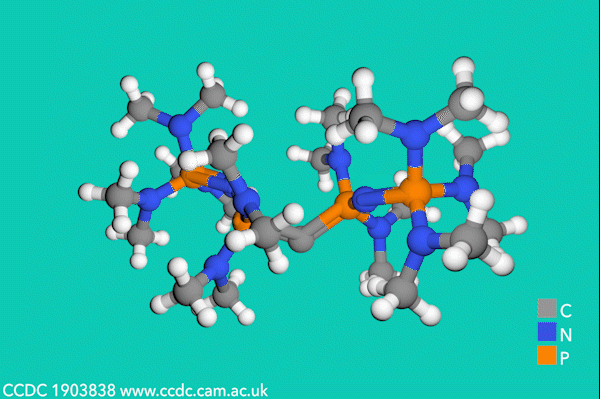
Now, Sundermeyer’s team has developed a new class of second-order carbodiphosphorane superbases with R3P–C–PR3 functionality, which demonstrate the highest basicity of any uncharged carbon base. The first experimental determination of the pKBH+ value for a carbodiphosphorane at 35.8 proved their record-breaking performance, being over two orders of magnitude higher than the previous record-holding phosphorus ylide (pKBH+ = 33.5 in THF).3
Despite side-reactions from the electron-rich phosphines causing initial synthetic challenges, the team formed these carbodiphosphorane superbases via an oxidative imination sequence. The preformed carbon–phosphorus bonds avoided the unwanted formation of phosphorus(iii) nucleophiles.
The team says that the convenient synthesis and low molecular weight of these compounds will help encourage their use in mainstream organic superbase catalysis.
References
1 S Ullrich et al, Chem. Sci., 2019, DOI: 10.1039/c9sc03565f (This article is open access.)
2 R Schwesinger and H Schlemper, Angew. Chem. Int. Ed. Engl., 1987, 26, 1167 (DOI: 10.1002/anie.198711671)
3 J Saame et al, J. Org. Chem., 2016, 81, 7349 (DOI: 10.1021/acs.joc.6b00872)









No comments yet