A collaborative effort between scientists has led to the isolation of a complex with two iodine(I)-silver(I) coordination bonds, opening up possibilities for other complexes with unconventional bonding.
Metal–metal bonds between metal cations are common, ranging from the manganese–manganese single bond in Mn2(CO)10 to the quadruple rhenium–rhenium bond in Re2Cl82-. Similar bonds between non-metal and metal cations are much rarer, often due to a mismatch in orbital energy which limits overlap.
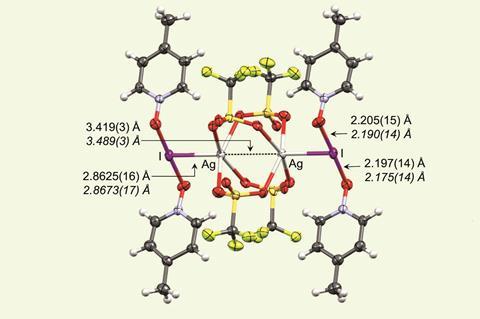
A group of scientists in Finland, Germany and Spain has now synthesised a compound containing such a bond. To do this, the researchers combined silver triflate with a pyridine N-oxide to create a polymeric silver compound, which reacts further with iodine. Within the resulting complex, two silver cations are bridged by four bidentate triflate ions, forming what is known as a paddlewheel structure. Each iodine cation – found at the heart of an unusual three-centre-four-electron complex with two pyridine ligands – forms a single orthogonal coordination bond to a silver cation.
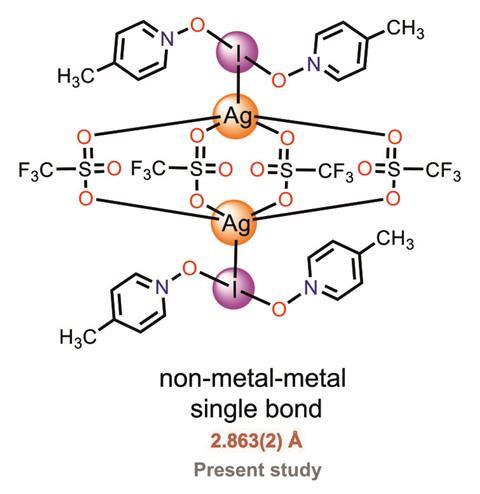
X-ray crystallography revealed that the iodine–silver bond length is 2.86Å, much shorter than the sum of the van der Waals radii of the cations (3.54Å). The reported bond length is similar to typical single metal–metal bonds. Computational analysis confirmed that there is significant covalent character within the iodine–silver bond, with the iodine(I) cation donating electron density to the silver(I) cation, owing to the increased electron density around the iodine from the N-oxide ligands.
The researchers note that future studies will explore similar complexes with other metals and various other ligand systems, with the aim of expanding the breadth of compounds with unusual coordination bonds.
References
R Puttreddy et al, Nat. Commun., 2025, 16, 7532 (DOI: 10.1038/s41467-025-62191-1)











No comments yet