Scientists in Australia and Germany have unveiled a new method for photolysing polyurethanes. Led by Christopher Barner-Kowollik at Queensland University of Technology, the group incorporated photosensitive o-nitrobenzyl moieties into a polyurethane, which enabled the polymer to be broken down upon exposure to UV light. Barner-Kowollik says the approach could aid the development of polyurethane-based degradable packaging and adhesives, and hopes it can be extended to other polymers.
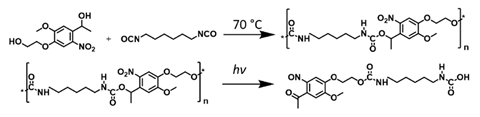
Polyurethanes are a large and widely used class of polymer, with applications ranging from industrial products such as sealants and adhesives, to drug delivery and biocompatible materials. The properties of polyurethane-based polymers are highly variable and can be tuned by varying the structure of their constituent monomers. However, the strong urethane linkage and often high degree of crosslinking in polyurethanes makes them a challenge to degrade without damaging the environment. Waste management of polyurethanes typically involves combustion, which is energy intensive and produces highly polluting gases, driving efforts to produce polyurethanes that are degradable by environmentally-friendly means.
The strategy developed by Barner-Kowollik and his co-workers degrades polyurethanes using UV light. Polyurethanes are typically prepared by reacting a diisocyanate with a polyol, and it is the latter that the group targeted to introduce photodegradability to the polymer. After incorporating a photosensitive o-nitrobenzyl moiety into their starting dialcohol, step-growth polymerisation with a diisocyanate formed polymers with photocleavable points at every other monomer unit. On UV irradiation, the o-nitrobenzyl group undergoes a Norrish II type reaction, ultimately resulting in heterolytic fission of the C–O bond and formation of a ketone-nitroso compound.
Through a combination of infrared spectroscopy and 1H-NMR techniques, the group were able to explore the degradation pathway of the o-nitrobenzyl moieties. Elizabeth Gillies, an expert in polymer chemistry and smart materials at Western University in Canada, found this aspect of the study particularly noteworthy. ‘Although o-nitrobenzyl groups have been previously incorporated into polymers to achieve photodegradability … [this study] will enhance our understanding of the degradation mechanism … [and] will assist in the future design of photodegradable polymers,’ she tells Chemistry World.
For Barner-Kowollik, the greatest challenge for future work lies in degrading bulk materials. ‘Our first approach consisted of the photodegradation of bulk polyurethane. Unfortunately, due to light scattering, the material remained unresponsive to our light treatment. Here, we overcame these limitations by invoking a layer-by-layer approach to degradation, which works particularly well in thin films. Future work will see longer wavelengths of light used to degrade more persistent plastic materials.’
Correction: The spelling of Christopher Barner-Kowollik’s name in the final paragraph was corrected on 26 February 2021
References
This article is free to access until 9 April 2021
C Petit et al, Chem. Commun., 2021, DOI: 10.1039/d1cc00124h











1 Reader's comment