New catalytic route opens the door to polymers made from boron and nitrogen
Synthetic organic polymers and plastics revolutionised the 20th century and helped shape modern-day society. But a new range of materials with useful properties could be in the pipeline thanks to a catalytic method for making ‘inorganic polystyrene’.
Polystyrene is an important material in today’s society with its uses ranging from a protective packaging material through to disposable cutlery. Its chemical structure, like the majority of other important synthetic polymeric materials, has a backbone of carbon atoms. To discover new materials with useful properties, researchers have tried to replicate these structures using inorganic chains, with silicone materials being a recent example. Now, Ian Manners and his team from the University of Bristol, UK, have made inorganic polymers out of boron and nitrogen.
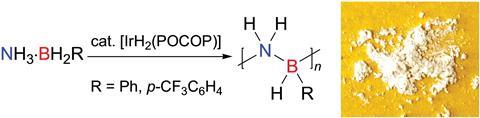
Hydrogen storage and transfer communities, in particular, have studied catalytic dehydrocoupling, where amine-boranes react together to yield hydrogen gas alongside a range of low molecular mass boron–nitrogen products. Adopting this process, Manners’ team has pioneered a procedure for producing longer-chain polymers based on a boron–nitrogen backbone. In this case, they used it to form a polymeric boron–nitrogen main chain with phenyl side groups – an inorganic analogue of polystyrene.
An iridium-based catalyst drives the process. ‘Finding a catalyst that yields polymer rather than depolymerisation products was a real challenge, as the energy barriers are much lower than for organic analogues,’ explains Manners.
While the new polymer is structurally similar to its carbon-based equivalent, its properties are very different. Its thermal stability was much lower, for example, meaning it degraded more readily than polystyrene, but the researchers could adjust this by varying the side groups. Manners suggests that these materials could have applications as ‘flame-retardant thermoplastics and elastomers, precursors to 2D hybrid materials, radiation-sensitive polymers, and as recyclable materials’.
‘Wow! Manners and his team have done it again,’ says Derek Gates, an expert in inorganic polymers from the University of British Columbia, Canada. ‘I find the ready degradation here to be quite intriguing, one could imagine taking advantage of this property to make easily degradable and recyclable plastics. I can’t wait to see what the future will hold for these exciting new materials!’
References
This manuscript is free to access until 13 December 2017
D A Resendiz-Lara et al, Chem. Commun., 2017, 53, 11701 (DOI: 10.1039/c7cc07331c)









No comments yet