Hotly debated historical brouhaha that centred on the element’s covalency may have been solved

After pioneering American radiochemist Glenn Seaborg voiced his suspicions that the elements after and including uranium constitute a distinct post-actinium series that he called the actinides, he had another hunch. In 1954 he suggested that americium – the next-heaviest element after plutonium and discovered by Seaborg’s team at the University of California at Berkeley in 1944 – uses its 5f orbitals to form partly covalent bonds with chlorine.1
Whether Seaborg was right has been hotly debated by actinide chemists ever since. Now a team at Los Alamos National Laboratory in New Mexico claims to have clear evidence that the americium–chlorine interaction does indeed have some covalent character, owing to the mixing of chlorine’s 3p orbitals with both the 5f and 6d orbitals of americium.
They are helping to make americium great again
Polly Arnold, University of Edinburgh
‘There are few things that can be so evocative as the notion of covalency, especially when invoked for actinides,’ says actinide chemist Steve Liddle of the University of Manchester. Understanding covalency in actinide bonding – how much, and using which orbitals – ‘is a key, fundamental concern, ongoing for decades and still fiercely debated’, he adds.
Enrique Batista, Stosh Kozimor, Ping Yang and their co-workers at Los Alamos set out to resolve the matter by studying the hexachloride anion AmCl63- using x-ray absorption spectroscopy (XAS), comparing the results to quantum chemical calculations of the bonding in the complex.2 XAS can provide information about the degree of mixing, and thus covalency, among the atomic orbitals. In these experiments the x-rays excite a 1s electron in chlorine, which can make a transition to the 3p orbitals, but only if they are partly mixed with orbitals of americium to create empty antibonding molecular orbitals. By comparing the spectra with calculations, the researchers could figure out how much mixing there is between the metal d and f orbitals and the chlorine p orbitals.
The measurements were made using x-rays from the Stanford Synchrotron Radiation Lightsource in California. It’s partly due to recent upgrades and improvements at such facilities, says Kozimor, that it has become possible to test the notion of actinide covalency in this way.
All in the mix
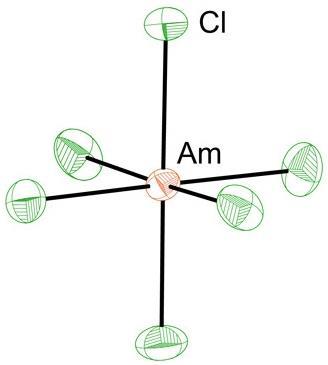
The researchers find that while the amount of d orbital mixing for americium and chlorine is similar to that of europium and chlorine – europium is directly above americium in the lanthanide series – the contribution to covalency from the americium 5f orbitals is greater than that of the europium 4f orbitals, probably because of their further extent in space and the better overlap between americium and chlorine valence orbitals. Kozimor says that ultimately it might be possible to control the mixing and covalency – and so, he says, to ‘selectively turn it on and identify new or unusual americium properties’.
It’s not clear that the findings will yet resolve the dispute about covalency to everyone’s satisfaction, though, as there are ongoing arguments about exactly how to define ‘covalency’ in orbital mixing: such mixing does not necessarily contribute to the bond energy.
Actinide–ligand bonding is far more than an academic issue because of the use of actinides in nuclear power and their presence in spent fuel. Separating them is crucial for the recycling and reuse of nuclear fuel – only a few percent of the fuel rods are used before they need reprocessing. Separation is accomplished by binding specific actinides to ligands in ion exchange techniques. ‘Even small differences in covalency can generate significant differences in behaviour, so this is important to understand,’ says Liddle.
‘The chemical separation of the highly radioactive minor actinides from nuclear waste relies on subtle chelation differences between these heavy, trivalent 5f cations from the lanthanides that are also present,’ says actinide expert Polly Arnold of the University of Edinburgh. ‘The best chelators have incredibly high selectivities, but it’s not clear why’.
Some have suspected that the chelators select more covalent cations, but this has been very hard to test experimentally, Arnold explains. Because they are so radioactive, the synthesis of these elements can only be done safely on tiny scales – which, she adds, is what makes the current results ‘a very powerful contribution’.
Besides going some way to clearing up a 60-year-old controversy, says Arnold, the new results can be considered to serve another end. To recycle a joke popular among actinide chemists, they are helping to ‘make americium great again’.
References
1 R M Diamond, K Street Jr and G T Seaborg, J. Am. Chem. Soc., 1954, 76, 1461 (DOI: 10.1021/ja01635a001)
2 J N Cross et al, J. Am. Chem. Soc., 2017, DOI: 10.1021/jacs.7b03755















1 Reader's comment