‘Deep eutomic’ mixtures allow easy access to unusual pharmaceutical polymorphs
A new type of solvent system that can easily encourage pharmaceutical compounds to crystallise into forms that were previously economically unviable has been developed by UK researchers.
The idea builds on the family of deep eutectic solvents – from the Greek for ‘easy melting’ – which are mixtures with much lower melting points than any of their components. They are widely used to run solution-phase reactions at lower temperatures than otherwise possible, saving energy and avoiding emissions of volatile solvents through evaporation.
Simon Hall and his team at the University of Bristol have created deep eutectic solvents by mixing pharmaceuticals like paracetamol with various volatile solids. Evaporating the volatile part from mixtures of different proportions can make the original compound crystallise in polymorphic forms that are usually much harder to produce.
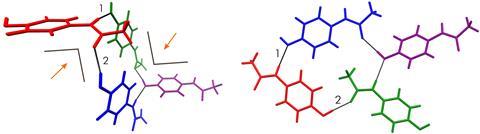
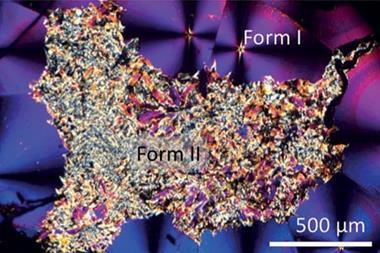
Pharmaceutical synthesis usually takes place in solution, and the final purification step is often crystallisation, promoted by evaporating the solvent. The structure that crystallises this way is usually the most thermodynamically stable. Unfortunately, this is also the least soluble and therefore likely less effective than metastable polymorphs. Paracetamol, for example, is sold in form I, even though an alternative polymorph – form II – works better. ‘You have to heat it to several hundred degrees, cool it in a certain way and have a template or additive to force it into form II,’ explains Hall, ‘It’s just not economically viable.’
Normally, Hall explains, a deep eutectic solvent would be created by mixing two solids together that are unrelated to the chemistry being performed. However, the team has ‘turned the concept of deep eutectics on its head’, Hall claims, by incorporating the compounds of interest as part of the eutectic mixture, then exposing the solvent to the atmosphere, forcing the volatile component to evaporate and the drug to crystallise. The researchers have named these self-destructing solvents ‘deep eutomic solvents’ from the Greek for ‘easily cut’.
The protocol allows very high control over polymorphism simply by varying the proportions of the two components in the mixture. The researchers demonstrate this by mixing paracetamol with phenol, then evaporating the phenol. Depending on the ratio of phenol to paracetamol, they obtain exclusively form I, exclusively form II or a mixture. They also demonstrate crystallisation of several other pharmaceuticals. ‘AstraZeneca is already looking at the kinetics of dissolution of these products and, for each drug, it’s now a case of looking at a range of volatile co-formers to make the deep eutomic and then [working out] which polymorph arises from which concentration,’ says Hall. ‘Perhaps the next step is to look at co-crystals, where you have two components in whatever drug or agrochemical you want to deliver. We’ve just filed a patent on that.’
Physical chemist Karl Ryder at the University of Leicester believes the technique will be ‘of interest to a range of different pharmaceutical manufacturers, especially if it allows the prescriptive selection of particular morphologies in particular drugs.’
References
J Potticary et al, arXiv:1902.08376










No comments yet