I only had a gun pulled on me once during my time at Chemistry World. It was on a chill October morning at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, and the military guards, still at their post from the Cold War with AK-47s at the ready, hadn’t been told about our cameras. It was only after some hasty negotiation that we were permitted entry and could see the machine – and meet the team – that discovered the five heaviest elements currently known.
It was all thanks to a partnership that had begun almost 30 years earlier, between two former adversaries. ‘It was a special, perhaps unique, long-running collaboration,’ recalls Mark Stoyer, a staff scientist at Lawrence Livermore National Laboratory (LLNL), US, and part of the American team that united with the Russians. It was a collaboration that was ‘extremely scientifically productive and fruitful’, he adds.
Making elements
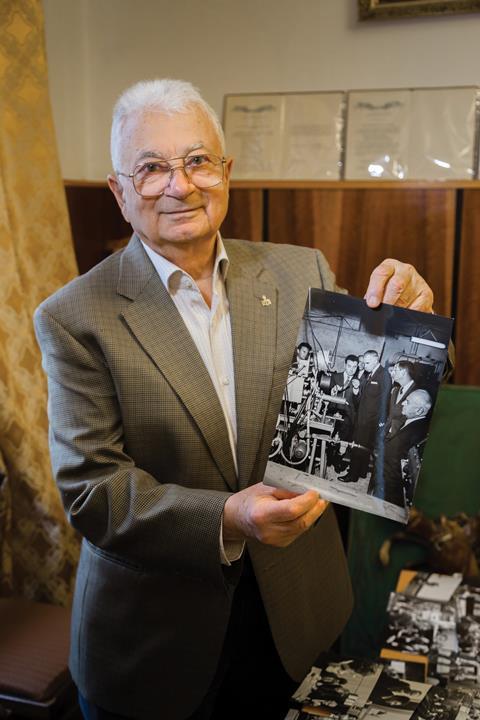
The reason we went to JINR was Yuri Oganessian – a man who was about to become the only living person with an element named after him. Warm, chuckling, almost grandfatherly, he greeted us in an expansive office, still filled with the drawings of his predecessor as head of JINR’s element discovery team, Georgy Flerov. It had been Flerov, Oganessian explained, that had started the unlikely partnership.
Since the 1940s, element discovery has pushed beyond the elements that exist naturally on Earth. Instead, they are created through nuclear fusion – smashing two atomic nuclei together, to create superheavy, radioactive elements. For 40 years, the US and USSR competed to add to the table, resulting in a stand-off that became known as the ‘transfermium wars’. By the 1980s, a new rival team at GSI Darmstadt in Germany had also started making elements. To gain the advantage once more, Flerov took an unprecedented step. In 1989, while at a conference, he spoke to LLNL scientist Ken Hulet and invited him to work with them. A new Russian–American team had been formed, in direct competition with the team at nearby LBNL.
Flerov died in 1990, but the collaboration blossomed under the leadership of his eventual successor, Oganessian. While the GSI discovered yet more elements, the JINR–LLNL group embraced a new technique, dubbed hot fusion, that would let them reach further. Usually, when nuclei smash together, there is too much energy, and the newly formed nucleus breaks apart in nuclear fission. The Germans used cold fusion, to make elements using as little energy from the accelerator as possible. This was reliable, but as the elements became heavier, the cross section – the probability of success – became smaller.
Timeline
Super heavy elements
1989
Collaborative beginnings
Georgy Flerov of the Russian Joint Institute for Nuclear Research approaches Ken Hulet of the US's Lawrence Livermore National Lab and proposes working together
1994-1996
Discovery
Elements 110–112 discovered at GSI Darmstadt, Germany
1997
An end to hostilities
The ‘transfermium wars’ end with IUPAC’s naming of elements 102–109
1999–2000
First JINR discoveries
Elements 114 and 116 discovered at JINR
2002–2004
Riken joins the race
Elements 115 and 118 discovered at JINR, and 113 at Riken and JINR
2009
Confirmation
IUPAC confirms the discovery by JINR/Livermore of elements 114, flerovium, and 116, livermorium
2010
Ununseptium
Element 117 discovered at JINR
2015
Confirmation continues
IUPAC confirms the discovery by JINR–LLNL of elements 115, moscovium, 118, oganesson, and (with Oak Ridge, University of Tennessee, Knoxville and Vanderbilt University) 117, tennessine; and the discovery of element 113, nihonium, by Riken
2022
Partnership suspended
JINR–Livermore partnership put on hold indefinitely due to the Russian invasion of Ukraine
2023
New collaborations
Lawrence Berkeley, with Oak Ridge and LLNL, announces new collaboration to create element 120
Hot fusion was different. The accelerator’s power was increased, making the chance of success greater, but fission more likely. To compensate, the team decided to use a beam of calcium-48, an isotope with eight more neutrons than usual. This allowed the newly formed nucleus to jettison the neutrons like a ship’s ballast, reducing the energy and, the team hoped, making the newly formed nucleus more stable.
The first successes
Oganessian’s guided tour of JINR was enlightening. There was, of course, the particle accelerator and its beamlines – a behemoth cyclotron that looked like a giant zinc battery in a clamp. But there were derelict buildings too, and jars of liquid nitrogen with baked bean cans for lids – a sign of constrained funding. Above all, there was a sense of excitement among the scientists: they were expanding the periodic table, seeking discoveries at the very edge of what was possible.
It was a thrill shared by their American counterparts, who I later visited in the warm, wine-growing Livermore valley just outside San Francisco. I met many of the LLNL researchers who travelled to JINR, most notably Stoyer and fellow element discoverer Dawn Shaughnessy. Coming from California, they had been shocked by the realities of Dubna: a town of brutalist architecture and Soviet murals on the banks of the Volga, with a single hotel (allegedly bugged) and no supermarket until a decade ago. Yet JINR had the best particle accelerator for element hunting in the world. Combined with the US team’s cutting-edge data analysis, and their secret weapon of calcium-48, the new elements flowed.
In the late 1990s and early 2000s, their technique had achieved a string of successes: first, elements 114 and 116 – confirmed in 2012, and named flerovium and livermorium to celebrate their historic partnership. Elements 113, 115 and 118 were sighted, too, as we reported in Chemistry World 20 years ago. But element 117 remained elusive. The problem was that, to create the element with calcium-48, the team needed a target made from element 97, berkelium. But there wasn’t any to be had. Large quantities of berkelium could only be produced in two nuclear reactors in the world, and as the element had no use, neither of them made it.

The problem was solved in 2010 at Oak Ridge National Laboratory – the site of the first permanent nuclear reactor, built for the Manhattan Project during the second world war. Still a major research site, Oak Ridge had one of the two reactors, and agreed to isolate enough berkelium for a target while it was producing element 98, californium, for commercial purposes. The JINR–LLNL partnership had gained a third member and element 117 soon followed, later to be named tennessine in honour of Oak Ridge’s home state.

A new rival
In 2015, enough evidence had accumulated that the remaining elements on the eighth row of the periodic table – 113, 115, 117 and 118 – had been made. Yet there was another twist in the tale, which would take me to Wako, a small city on the edge of the Tokyo subway. Outside the station, bronze plaques for each element of the periodic table led me, like a trail of breadcrumbs, to the gates of the Riken research institute. Here, in a surprise coup, a Japanese team had been recognised with the discovery of element 113, using the older cold fusion technique. ‘Discovering an element was a dream from Japan’s history,’ Hideto En’yo, director of Riken’s Nishina Center, told me. ‘A dream to recover from a mistake … for Japan this has been a century-long project.’

The mistake was Masataka Ogawa’s claim to have made element 43, which for several years was known as nipponium before it was struck down. Under the direction of Kosuke Morita, the Japanese team spent a total of nine years trying to find three atoms of element 113 to confirm their discovery, only obtaining enough time with the accelerator after other experiments were shut down in the wake of the Fukushima nuclear incident in 2011. The result paid off – and Japan, and Asia, was recognised with its first element on the periodic table. Unable to use nipponium again as a name, it was instead named nihonium, after nihon, an alternative word for Japan.
Yet Riken’s fillip to Japanese prestige does not subtract from JINR–LLNL’s astonishing achievement. And for many, the collaboration became more than a career highlight: it gave them a unique place in history. Shaughnessy and Stoyer have overtaken Marie Curie as the most successful women to discover elements, both claiming five; at Oak Ridge, Clarice Phelps similarly made history as the first black woman to discover an element – tennessine – an achievement that has catapulted her to appearances on national TV and radio across the US. The city of Livermore has even created Livermorium Plaza in the team’s honour.
Oganessian’s name is also immortalised on the periodic table, along with that of the man who set up the collaboration, as flerovium and oganesson. ‘It’s difficult to say how I feel,’ Oganesson told me, when I asked what it felt like to be the only living person with an element named after him. ‘It was proposed by my collaborators. All of these people who were involved and came to this conclusion … it’s an honour for me, but it’s my friends and colleagues who wanted to express it’. For his team, it is richly deserved. Oganessian is not a principal researcher in the traditional sense; they describe him as the ringmaster of a circus or director of a film – a man whose gifts are creativity and leadership, rather than solely pure science. Despite being in his 90s, he is still searching for more elements.
In 2019, the JINR and LLNL researchers celebrated 30 years of collaboration. They already had plans for yet more elements, too. While calcium-48 couldn’t be used (to make element 119 it would need to be shot into an einsteinium target, an element that has only ever been produced in microgram quantities), they had an alternative. JINR had a new, better machine – the Superheavy Element Factory – which would fire a titanium-50 beam at a californium target made at Oak Ridge, to produce element 120. Success, they predicted, would come within five years.
Fate had other plans.
Going dormant
‘Through no fault of the teams,’ Stoyer says, ‘the Covid-19 pandemic and the political situation [caused by Russia’s invasion of Ukraine] curtailed travel between the countries’. Without the ability to travel, the US and Russians couldn’t collaborate as effectively, and with the breakdown in diplomacy between the east and west, US government laboratories can’t supply the Superheavy Element Factory with the target material. ‘The situation is actually not easy in the current turbulent environment,’ Oganessian told Chemistry World last year. ‘We cannot cooperate with [LLNL] and Oak Ridge because … JINR is located in Russia.’
As suddenly and surprisingly as it began, the JINR-LLNL partnership appears to be over.
Today, the focus for new elements has shifted. Oak Ridge continues to work with Riken, putting the Japanese firmly in the lead for the discovery of element 119 by firing a vanadium beam at curium targets. And, last year, the US government announced plans for a new hunt for element 120, led by JINR’s cold war rivals Lawrence Berkeley – which had abandoned element discovery in the early 2000s – and with support from Oak Ridge and LLNL. It’s here that most in the element-hunting community put their hopes. Other possibilities include GSI Darmstadt once work on a new synchrotron is complete, and a team at the Ganil accelerator in Caen, France.
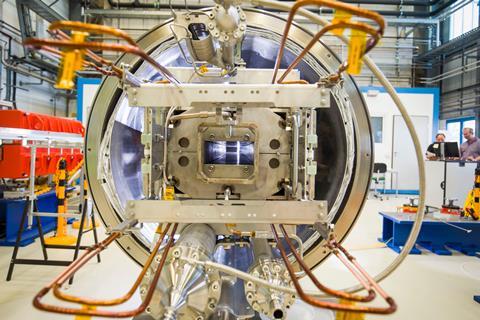
Yet the legacy of 30-years of shared successes and friendships is unlikely to vanish overnight. ‘I prefer to think of the collaboration as dormant,’ Stoyer says. ‘Collaborative ideas are marinating for when it does become easier to work together again in the future.’
Even if it doesn’t come to pass, though, its legacy is something that none of the researchers, including Stoyer, regret. ‘I can honestly say that I am thankful for the JINR–LLNL collaboration. And especially thankful for the many creative, hard-working and talented individuals involved.’
















No comments yet