Earlier this year, a team of chemists created extraordinary electrical conductance in polystyrene, a usually non-conducting polymer, without altering its chemical makeup. Another group lowered the melting temperature of DNA so that it could fold into ‘origami’ structures at lower temperatures – again, without changing the molecules’ structure. And yet another research team managed to decrease the polarity of long-chained alcohol solvents without chemical modification.
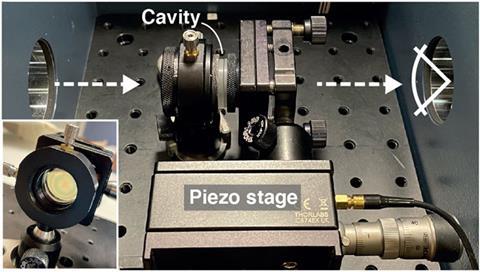
Behind these astonishing results is a seemingly simple setup: a small box, only a few micrometres wide, with mirrored walls. In this optical cavity molecules behave strangely: reactions can be sped up or slowed down, product distributions can be altered, polarity or electrical conductivity changed. The cavity allows scientists to tap into the power of the vacuum field, the transient quantum fluctuations that are baked into the makeup of the universe.
But there’s still no consensus on the mechanism behind the phenomenon and no way to predict which reactions are susceptible to the vacuum field’s influence. This, combined with failures to reproduce a number of results, has made some researchers sceptical that the cavity’s effect on chemical reactivity is real at all. Researchers are now trying to bridge the gap between theory and experiment to understand what is really going on in a cavity.
Space, the final frontier?
Empty, dark space is not truly empty, nor dark. It’s teeming with ‘virtual’ particles that emerge from random quantum fluctuations. Photons and other particles pop into existence, seemingly out of nothing, and disappear again. This vacuum field sounds a little like a return to the long-discarded aether theory, but it becomes apparent in other quantum phenomena such as the Casimir effect and zero-point energy.
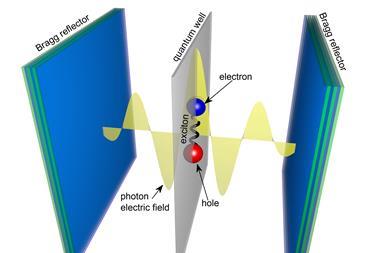
The way to access the vacuum field’s power for chemistry is by capturing it inside a cavity. As physical chemist Marissa Weichman from Princeton University, US puts it: ‘The cavity field confines light.’ Only photons whose wavelengths fit an integral multiple of the gap between the mirrors can exist inside the cavity.
The trapped field of light, or cavity mode, can be tuned to resonate in energy with one of the vibrations of molecules placed into the cavity. In the same way atomic orbitals interact and mix to form molecular orbitals with entirely new properties, the cavity’s light field couples with the molecules’ vibrations to form new hybrid light–matter states. These vibrational polaritons inherit the wavelike nature of light while maintaining the molecule’s structure. And they often have dramatically different properties to their parent molecules – so dramatic that they could be likened to a new state of matter.
A molecule can be thought of as a complicated mechanical system made of vibrating bonds and angles
Oriol Vendrell, a theoretical chemist at Heidelberg University, Germany, explains that a molecule can be thought of as a complicated mechanical system made of vibrating bonds and angles – some with softer and some with stiffer springs. ‘When you put the molecule in a cavity, it’s like adding new springs to the molecule.’
The basic idea of polaritonic chemistry revives a proposal from the mid-20th century. Bond- or mode-selective chemistry was meant to excite certain vibrations within a molecule using laser light of a specific wavelength until it breaks at a specific point and initiates a reaction. ‘These are really beautiful ideas, but they never really took off,’ Weichman says.
As a 1982 paper entitled ‘Is bond selective chemistry possible?’ puts it: ‘Although there have been many attempts using conventional CO2 lasers to carry out bond-selective reactions by infrared multiphoton excitation, none has been successful.’ The problem is that energy pushed into a vibrational mode doesn’t stay neatly confined to it. It quickly redistributed across the entire molecule. ‘You end up just kind of heating up the system instead of doing something specific,’ Weichman explains.
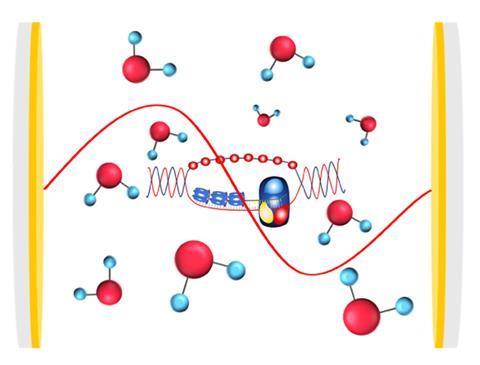
In 2012, a team around Thomas Ebbesen at the University of Strasbourg, France, showed for the first time that the vacuum field can change the rate of a chemical reaction: the photoisomerisation of a spiropyran derivative slows down inside a cavity. Many other experiments since have shown changes in rate and product distribution, including in named reactions, biochemical transformations and enzymatic reactions.
This is totally different from what we usually think about catalysis
Wei Xiong, University of California, San Diego
Cavity catalysis could steer reactions down different paths without changing temperature or pressure, without adding any reagents or altering the reactants’ chemical structure. ‘This is totally different from what we usually think about catalysis,’ says Wei Xiong from the University of California San Diego, US.
So far, experiments could only show altered reaction rates or product distributions. Angel Rubio from Germany’s Max Planck Institute for the Structure and Dynamics of Matter, says that the first demonstration of vacuum-field catalysis producing an entirely different product would be a real breakthrough. Cavity-controlled chiral chemistry in particular, he adds, would have ‘tremendous implications’.
Most experiments have demonstrated reactions slowing down, which is arguably not as useful as speeding them up. But it might help to shut off unwanted side reactions or increase selectivity towards one of several possible products. ‘It has the potential to influence energy generation and storage,’ says Blake Simpkins from the US Naval Research Laboratory. Slowing down the degradation of organic photovoltaic materials, he points out, would be very useful.
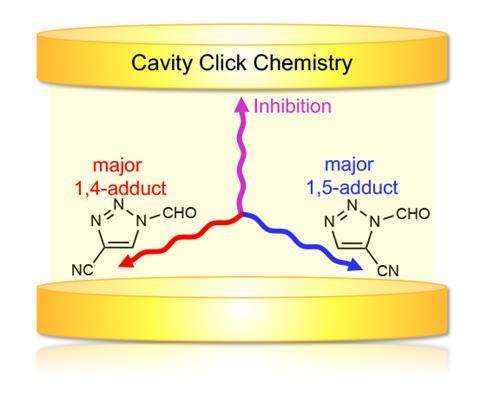
Several theoretical studies have given tantalising glimpses of what might be possible. Rubio’s team, for example, showed that a click reaction run in a cavity doesn’t need a catalyst. And if the reactants could be aligned in a particular way, the reaction could be selective for only one of the two possible click products. ‘I think the dream is that you’d be able to integrate these sorts of cavity devices into microfluidic flow reactors and actually use them in real industrial thermal reactions,’ says Weichman.
Theory remains elusive
Having a new knob with which to tune chemical properties could revolutionise synthesis. But how to operate this knob remains unclear. There’s no single accepted theory that can fully explain experimental observations.
A team led by Simpkins and Felipe Herrera from the University of Santiago, Chile, recently showed that the vacuum field can slow down the alcoholysis of phenyl isocyanate with cyclohexanol by 80%. ‘It’s one of the most compelling data points that you can alter chemical reaction rates in cavities,’ says Weichman. The team suggests that the cavity is affecting the population of vibrational levels. ‘It’s almost as if things are cooling down a little bit,’ say Simpkins. ‘It’s almost like we’re siphoning off a little bit of vibrational excitation, and that redistributed system now has less excitation to drive it toward the reaction.’
You have to compare the state of the field today with transition state theory in the 1950s
Felipe Herrera, University of Santiago
As with some other proposals, ‘what is missing, I think, is a direct connection between these hypotheses and an actual rate’, says Vendrell. What researchers agree on is that vacuum-field catalysis is not about reaction energetics. ‘I think what most people in the field would say at this point is that … the way that vibrational energy flows in the system is somehow altered in cavities,’ Weichman says.
Most reactions or compounds that are susceptible to the vacuum field have been discovered by coincidence. None of the theories currently have predictive power. There do seem to be some general trends: Rubio says that reactions that interact strongly with the cavity usually have a large dipole moment.
Herrera points out that it’s still early days. ‘You have to compare the state of the field today with [transition state] theory in the 1950s,’ says Herrera. ‘People could not calculate the reaction rate constant. They can do it trivially now.’
And there’s a fundamental paradox that needs to be addressed: logically, the cavity effect should be too small for each molecule to make a difference. In calculations, when a single molecule is placed inside a cavity and the effect of the light–matter coupling is put into that one molecule, ‘then things happen’, says Vendrell. But a real cavity contains an enormous number of molecules. The cavity effect becomes so thinly stretched out over all of them that it is essentially non-existent for each individual molecule. ‘We don’t understand how these very tiny couplings per molecule may end up having an effect on the total reaction rate,’ Vendrell says.
Simpkins suggests that disorder might be key to solving the mystery. Even chemically identical molecules have different local environments. ‘The disorder, these little sub-populations, they’re able to harness the cavity-induced effects more effectively than if all the molecules were identical,’ he explains. ‘The impact remains localised on a fewer number of molecules and therefore it’s larger.’
Replication complication
The lack of theoretical consensus, combined with lingering concerns about reproducibility, makes some researchers sceptical that the effect is real. Xiong says that at this point he is ‘not confident’ to claim that dark cavities really alter chemical reactivity. ‘I would be confident if one day I can reproduce someone’s result.’
In 2021, he and a colleague attempted to reproduce an ester hydrolysis that was reported to be an order of magnitude faster inside a cavity. Even after getting in touch with the team that originally reported the experiment and tweaking their setup accordingly, they failed. ‘There must be some really subtle condition that was overlooked between our attempt and their result,’ Xiong says. Another team, led by Noel Giebink from Pennsylvania State University, US, was unable to reproduce an experiment that reported a 100-fold enhanced rate of a cyanate ion hydrolysis.
‘The big issue is that there really aren’t established best practices for how to build the cavities, how to interrogate them, how to how to ensure that there are no artefacts contaminating your data, ’ says Simpkins. The tiny, nanolitre volume of the cavity means that even small inconsistencies can have large consequences. ‘I think what’s really hard is the analysis,’ adds Weichman. ‘Unless you’re very, very careful, I think you could also trick yourself into thinking you’re seeing reactivity that you’re not.’
When Weichman and her team examined a simple, highly exothermic reaction between cyanide radicals and chloroform, they found a conspicuous absence of cavity effects. This doesn’t mean the cavity never has an effect on any reaction, but they argue that reporting null results is crucial for understanding the mechanism.
Part of the problem is that theory and experiment haven’t quite met yet, Weichman suggests. Many theoretical approaches simplify too much and many experiments use systems too complicated to be treated theoretically. ‘But I think there’s mutual goodwill to try to bridge that gap,’ she says. Her team is part of that effort, working on simple, clean systems such as gas-phase polaritons that are easier to simulate computationally.
Simpkins thinks that some researchers might be hesitant to get into the field because of the reproducibility issues. But for others, it might be a call to action. ‘It’s actually really a rare time in a field for there to be results that are so exciting and really not fully understood yet,’ says Weichman. ‘That’s, I think, why a lot of people are flocking to this field, because there’s a lot of work to do, and a lot of questions that are ripe for study.’













No comments yet