At first glance, cyclocarbons look almost boring: rings of carbon atoms that can be drawn either with alternating triple and single bonds, or all double bonds. But their simplicity is deceptive, as these compounds remained impossible to make for decades. Only in the last few years have chemists managed to push the limits of synthesis far enough to gain insights into these new molecular forms of pure carbon – some of which could change how we define aromaticity.
The idea of cyclocarbons as another carbon allotrope has long fascinated chemists ‘Most chemists are drawn to beauty and to symmetry,’ says Rik Tykwinski, a physical organic chemist from the University of Alberta, Canada. As far back as the 1960s, Nobel laureate Roald Hoffmann used cyclocarbons as a test of his aromaticity calculations. Back then, they were purely theoretical molecules and little was known about their bonding, geometry and aromaticity. Predictions of these properties have often been contradictory and controversial. ‘You do a calculation by one method, and it predicts this, you do a calculation by another method, it predicts that, or the opposite,’ Tykwinski explains. ‘Until you make the molecule, you can’t answer these questions.’
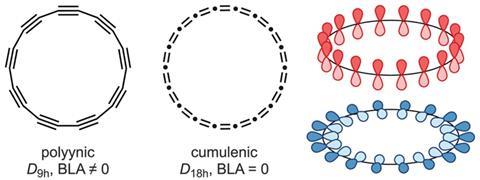
During the 1980s, evidence emerged that cyclocarbons actually existed when they were spotted in gas-phase experiments. In 1989, François Diederich and his team at the University of California, Los Angeles presented the first rational synthesis of cyclo[18]carbon (C18) in the gas phase.1 At the time, buckminsterfullerene had just been synthesised and some chemists thought cyclocarbons could be another stable allotrope. But it didn’t turn out to be quite as simple – it would take another 30 years until a including the University of Oxford’s Harry Anderson, once a postdoc in Diederich’s group, would make C18 in the condensed phase in experiments requiring cryogenic temperatures and stabilisation of the material on a crystalline surface.2
Over the last few years, a friendly competition between the only two cyclocarbon-making teams in the world has propelled the field forward to new records. Wei Xu‘s group at Tongji University in China currently claim the smallest cyclocarbon – C6, which they reported in a preprint in October.3 And in November, a group co-led by Anderson, his postdoc Igor Rončević, and Zurich-based IBM researcher Leo Gross posted a preprint reporting the synthesis of C13, the first of its kind with an odd number of atoms.4 ‘It’s equal parts ingenuity, dedication and technical advances,’ Tykwinski says.
Researchers have started to uncover unexpected aromaticity in some cyclocarbons, which is calling into question our definition of the term. And although all cyclocarbons so far have been made on surfaces, we might see the first off-surface structure this year and even witness their application in the synthesis of new, as yet unexplored, carbon materials.
The design cycle
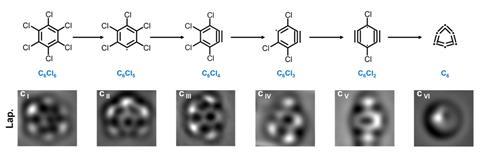
At the heart of the recent discoveries are advances in single-atom manipulation, specifically atomic force (AFM) and scanning tunnelling microscopy (STM). At ultra-low temperatures, precursor molecules are placed onto a metal single crystal coated with a few atomic layers of inert sodium chloride. Voltage pulses from an atomically thin microscope tip are then used to convert the precursor into a cyclocarbon, either by splitting off ‘masking’ groups such as carbon monoxide or chloride, or by breaking internal bonds. Seeing the molecule essentially form in real time under the microscope, ‘that’s the fun part’, says Gross.
A lot of clever thinking goes into designing the precursor. It needs to be stable enough to survive the sublimation process that transfers it onto the surface but unstable enough to ‘unmask’ the cyclocarbon through tip-induced reactions. Often, it’s hard to predict whether this will work in the way it’s envisaged – if it doesn’t, a lot of time can be wasted on this step. ‘The synthesis of the C18 precursor took a year,’ Gross recalls. ‘The precursor was very unstable, so we had to ship it on dry ice and be very fast with the preparation. And really, I was very sceptical that the on-surface step, where we dissociated several carbon monoxide molecules, would work.’ In this instance, the gamble worked out and the team could lay their eyes on the first-ever cyclocarbon made in the condensed phase. The experiment is then repeated tens of times to ensure the synthesis actually works. Still, ‘once we have two or three observations, then we’ll get excited’, says Anderson.
Surface synthesis has allowed chemists for the first time to investigate cyclocarbons’ sometimes surprising aromaticity. Cyclocarbons have two pi systems, one in the plane of the molecule and one perpendicular to it. ‘We can have both pi systems aromatic, that’s fine,’ explains Rončević , referring to examples such as C18. ‘You can have both pi systems anti-aromatic, that’s also fine,’ he says. This is the case in C12, for example. ‘But then we can also have a situation in which one of the pi systems is aromatic and the other is anti-aromatic.’ This occurs in C16.
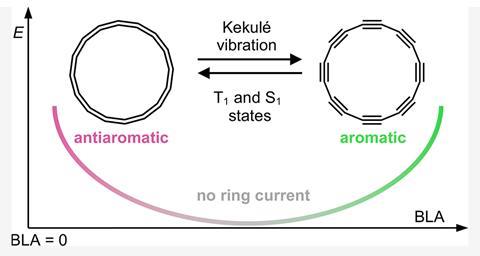
C16’s ground state is doubly anti-aromatic – an uncomfortable situation that causes the molecule to be strongly distorted from what would otherwise be a symmetric ring with all equal bonds.5 But with a small push of energy, the molecule can enter an excited state in which the spin of one of its pi electrons is flipped.6 This makes one of the pi systems aromatic while the other remains anti-aromatic. ‘One pi system is pushing the molecule towards being nice and symmetric and round and aromatic, and the other is pushing it to distort and try to escape its anti-aromaticity,’ Rončević explains. In this strange, finely balanced state ‘the concept of aromaticity becomes a bit funny, because tiny changes in the geometry can change the weights of one pi system over the other’, he says.
The team calls this situation ‘mixed’ aromaticity, a name that doesn’t quite do justice to how badly it fits into our understanding of aromaticity. Small changes to a molecule’s geometry shouldn’t drastically change its aromaticity, yet in C16 that is exactly what happens. ‘This is in a way a re-evaluation of our criteria for aromaticity,’ Rončević says. ‘If a model can’t describe such a simple molecule, then is it a good model at all?’
The next targets
For Anderson, the next big goal is to make cyclocarbons stable under ambient conditions. As long as the molecules are on a surface, ‘you can’t discount the fact that the surface has some role in [their] geometry’, explains Tykwinski, who says he has a bet with a friend over whether single-atom manipulation actually qualifies as synthesis. ‘He said it doesn’t qualify until you can put it into an NMR tube and see that there’s only one peak because there’s only one kind of carbon,’ Tykwinski says.
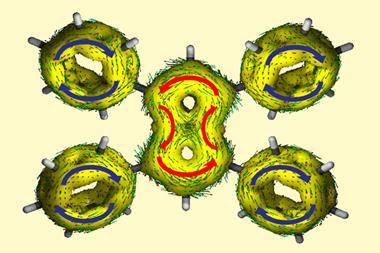
Anderson and his team are working on incorporating cyclocarbons into catenanes, macromolecules consisting of interlocked rings. ‘The idea is that you thread rings around the cyclocarbons, so you have many interlocked rings that keep the molecules away from each other and molecularly encapsulate them,’ he explains. ‘We’re not certain yet, but we think we’ve got species that are lasting for tens of minutes under ambient conditions. If we can just make them a little bit more stable and get them into a crystal – a crystal structure of a cyclocarbon would be really nice. I’m hoping [this] year, but you never know.’
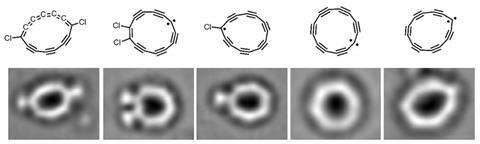
Researchers agree that there probably aren’t any large-scale applications for cyclocarbons on the horizon. However, cyclocarbons could be used to make new kinds of carbon structures including very large rings. The first hint that this might be feasible came from the work on C13, which unexpectedly fused with a neighbour to make C26. ‘We expect that cyclocarbons may be precursors to some unique two-dimensional carbon materials, like graphdiyne,’ says Xu . Proving this, he says, would be the next big achievement for the field.
‘Alternatively, one could try using cyclocarbons as synthons to add them to another molecule – that’s a completely unexplored type of chemistry,’ suggests Rončević. ‘We are also interested in whether we can use these molecules as artificial molecular machines or as logic elements, which we would steer with single electron charges, making them very power efficient,’ Gross adds.
Even if cyclocarbons don’t find immense practical use, Rončević points out that they deepen chemists’ understanding of basic concepts such as bonding, aromaticity and light-matter interaction. ‘Almost everything we learn about cyclocarbons is a surprise, because we really don’t know anything about them experimentally,’ says Tykwinski. ‘For me, the biggest surprise was that they could actually be made and seen.’
Correction: This story was updated on 24 January 2024 to clarify the type of distortion seen in C16.
References
1. F Diederich et al, Science, 1989, DOI: 10.1126/science.245.4922.1088
2. K Kaiser et al, Science, 2019, DOI: 10.1126/science.aay1914
3. W Xu et al, Res. Sq., 2023, DOI: 10.21203/rs.3.rs-3411973/v1
4. F Albrecht et al, 2023, DOI: 10.26434/chemrxiv-2023-ddrh7
5. Y Gao et al, Nature, 2023, DOI: 10.1038/s41586-023-06566-8
6. I Rončević et al, J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c10207





![Cyclo[48]carbon [4]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/6/3/542463_indexady6054_articlecontent_v2_18june3_70174.jpg)







No comments yet