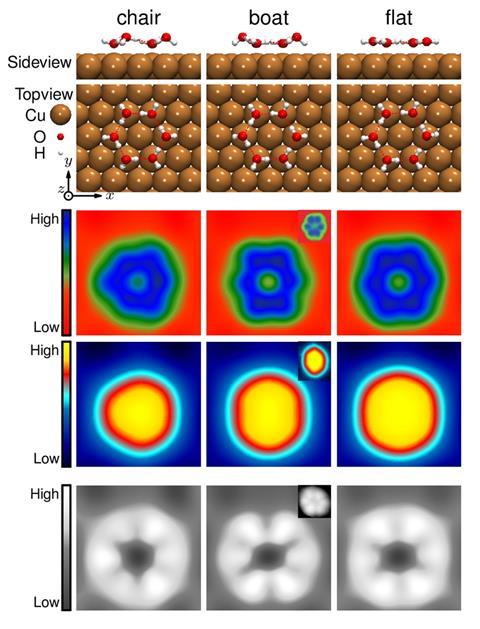
Water molecules arrange themselves into boat-shaped hexamers on metal surfaces. That’s the conclusion of a theoretical study that claims to have solved a long-standing discrepancy between experimental results – which seemed to show flat hexamers – and theoretical predictions of a chair conformation.
Water is weird. Its unusual properties have led some researchers to think of it as two different liquids instead of one homogenous material. In 2002, chemists discovered that thin water layers form regular hexamers on hydrophobic metals like copper at low temperatures. In scanning tunnelling microscope (STM) images, these structures looked flat. But several teams’ theoretical calculations later showed a chair-like adsorption configuration to be more stable than a flat hexamer.
A team of scientists in China and Sweden now thinks that it has solved the water puzzle, showing that a boat-shaped hexamer not only matches the experimental result but is in fact lower in energy than the chair conformation.
The team simulated STM images through ab initio molecular dynamics. Only for the boat configuration did they show the same elongated, C2 symmetric hexagon seen in experiments. Further calculations confirmed that the boat configuration is an electronic global energy minimum, meaning it is more stable than either the chair or the flat hexamer.
A better understanding of how water interacts with surfaces could help researchers design materials whose properties change in the presence of water.
References
S Duan et al, J. Am. Chem. Soc., 2020, 142, 15, 6902 (DOI: 10.1021/jacs.0c01549)











No comments yet