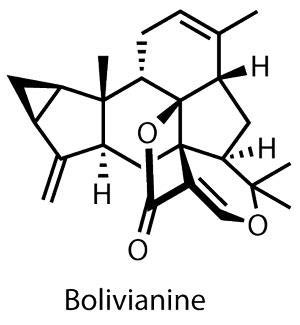
Handily, the isolation team has suggested a potential biosynthesis pathway, recognising elements of other natural products in its construction. Valérie Jullian’s group at the University of Toulouse, France, proposed that onoseriolide (a sesquiterpene found in the bark of related plants) could provide three isoprene units’ worth of the core, sharing the 3,5,6-carbocyclic system of bolivianine, while the remaining two isoprene units could be derived from ß-E-ocimene via a fairly lengthy biosynthesis.
However, Bo Liu and his team at Sichuan University in China have a slightly different take. They propose that this unusual terpenoid’s biogenesis hinges on an enzymatically catalysed Diels–Alder cycloaddition.2 While synthetic chemists have been combining dienes with dienophiles for over a century, the presence of a [4+2] cycloaddition in nature has been somewhat more controversial. Typical Diels–Alder type reactions generally require a significant thermal stimulus or a potent catalyst – neither of which were presumed possible in biological systems. However, recent findings seem to confirm the existence of a biological partner to the synthetic chemist’s favourite tool.3
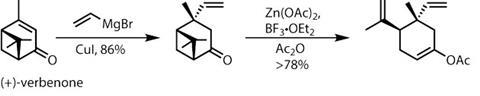
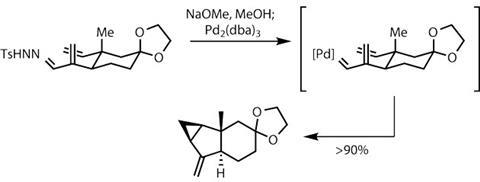
It was then time to test the team’s biosynthetic hypothesis. Attempting a direct cycloaddition with onoseriolide and the favoured diene partner, ß-E-ocimene, failed. Even strong heating would not induce a reaction – a significant setback. After re-examining the system, Liu and his team decided that the electronic partnering may not have been ideal, and that a slightly elaborated onoseriolide might react. Happily, this worked in their favour.
Oxidising the alcohol attached to the recalcitrant furan produces an aldehyde, activating the furan for cycloaddition (figure 3). After this simple modification, adding ß-E-ocimene becomes highly successful, initiating a cascade reaction which creates three rings, four carbon–carbon bonds and five new stereocentres – quite a day in the lab! The cascade begins with a Diels–Alder reaction, which sets up an intramolecular hetero-Diels–Alder between the alkene at the other end of the ocimene and the a,b-unsaturated aldehyde.
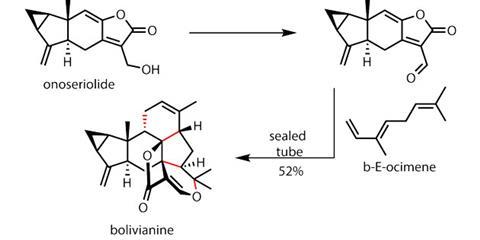
Completing the synthesis of a target as complex as bolivianine through traditional synthetic means is challenge enough, but using a biomimetic approach is both challenging and rewarding. The team has not only completed an extremely intricate target, but made an intriguing biosynthetic proposal more credible.
Paul Docherty is a science writer based in Reading, UK











No comments yet