Treating the bacteria that live inside us can improve our own health. Clare Sansom meets our tiny friends
All disease begins in the gut. That saying is attributed to the ancient Greek physician Hippocrates, who gave his name to the oath that doctors still use today. It is undoubtedly a simplification, but an enormous range of diseases, many with no digestive symptoms, have been associated with the gastro-intestinal (GI) tract. And some unusual therapies have arisen from there, too. In China, the use of healthy human faeces to treat some digestive disorders dates back over 1500 years. Much later, in the 16th century, Ming dynasty physician Li Shizhen gave patients with these problems a mixture of stool and water he named ‘yellow soup’ to drink.
Li is unlikely, however, to have known the active agent in his preparation: the microbes that live in our guts and that are therefore excreted in faeces. It would have been perhaps a century later when the so-called ‘father of microscopy’, Antonie van Leeuwenhoek, turned the microscope of his own design on a sample of his stool and observed that it contained microbes, which he had termed – based on the Latin for ‘tiny animals’ – ‘animalcules’ (in a rough translation from the original Dutch ).
We now know that each of us is, in some respects, more microbe than human. Each human body is colonised by as many as 100 trillion microbes: rather more, in fact, than our own cells, and contributing about 2kg to an adult’s body weight. There are microbial colonies on and around many organs but the gut, and particularly the colon (or large intestine) carries the greatest microbial load. The greatest proportion of these are bacteria and viruses, but the colonies also include large numbers of parasites and single-celled eukaryotes. The bacterial fraction is the best understood: we know so little about 80% of the viruses that inhabit our guts that they have been collectively termed ‘viral dark matter’.
A sequence of success
Before the genome era, however, we understood less still about the bacteria in our guts. ‘If you can’t isolate and then sequence the [genomes of] bacteria you can’t study their biology fully,’ explains Mike Romanos, chief executive of Microbiotica, a biotechnology company based in Cambridge, UK. And sequencing the genomes of all the bacteria in the human gut has proved to be a much harder challenge than sequencing those of organisms that live freely and can be readily cultured. This task only became tractable once Trevor Lawley from the Wellcome Trust Sanger Institute near Cambridge had perfected techniques for the deep culturing and sequencing of heterogeneous microbial samples obtained directly from the environment. This work led directly to Microbiotica’s founding to exploit this knowledge in the development of novel microbiome-based biomarkers and therapies. ‘In our case, the environment from which the samples are taken for analysis of their metagenomes, or for culturing, is the human body: most often, the human colon,’ says Romanos.
If you can’t sequence the bacteria you can’t study their biology fully
One of the most remarkable features of the human gut microbiome is its diversity. Microbiotica has developed the largest database of complete reference sequences for gut bacteria anywhere in the world. ‘Our database currently has full genome sequences of about 2500 species and should reach 5000 later this year; this is effectively a blueprint of the human microbiome and enables Microbiotica to precisely characterise the bacteria in patients,’ he adds.
Outside the fairly large group of core microbes found in the gut of most healthy adults, the complete microbiome of each individual is both constant over most of the lifespan and subtly different from others. Only large and long-lasting changes, such as developing a chronic or life-limiting illness or switching to a vegan diet are reflected in lasting changes to the microbiome. Generally, the healthiest microbiomes – those that will help to digest, without difficulty, the widest variety of foodstuffs – are the most diverse.
You are what you eat
The best way of fostering such a microbiome is to do exactly what we are all constantly nagged to do: eat a varied diet with a high proportion of fruit and vegetables and a low proportion of highly processed food. ‘Processed food is readily digested in the upper gut, so little passes down into the colon to provide nourishment for the bacteria that live there, and they starve,’ says Karen Scott, a research fellow at the University of Aberdeen in Scotland. ‘But individuals with similar healthy diets will have different healthy microbiomes: it’s best to define a healthy microbiome in terms of enzyme function – what reactions the bacteria are, together, able to catalyse – than in terms of what species are there.’
Our microbiomes don’t start off that way, however. If a diverse diet leads to a diverse microbiome, the reverse is also true, and infants before they are weaned – with the least diverse diets of all – have the least diverse microbiomes. Babies are born with relatively few gut bacteria, and as they start to take in milk, their GI tracts become colonised with bacteria from one genus in particular: the bifidobacteria (bifids). One species, Bifidobacterium infantis, predominates in the guts of breast-fed babies, and we now believe that these bacteria play an important role in ‘priming’ a baby’s immune system to respond to infection safely and effectively in later life. The so-called ‘baby biome’ study, published in Nature in 2019, has confirmed what many suspected: 21st century life is bad for infant microbiomes. Bifids are most dominant in babies delivered vaginally and fed exclusively on breast milk, both of which occur relatively infrequently in many Western countries.
Individuals with similar healthy diets will have different healthy microbiomes
As children grow and, hopefully, try different types of food, their gut microbiomes develop ‘adult-like’ characteristics. From late childhood on, an individual’s microbiome should stay relatively constant – with travel or illness bringing only transient changes – until old age. Even then, it is illness as much as age itself that drives the changes. A healthy octogenerian who maintains a diverse diet may have a microbiome with ‘younger’ characteristics than someone a generation younger with an underlying health condition that, perhaps, limits the food options open to them.
Just as a healthy diverse gut microbiome contributes to the health of an individual, so disruptions in the microbiome – so-called microbial dysbiosis – contribute to a wide variety of diseases, and not only the most obvious ones. The list of diseases with a known or suspected microbiome component is growing all the time and includes neurodegenerative diseases and cancers as well as gut and metabolic disorders. And a wide range of therapies that target the microbiome have been and are being developed, although most of these are considered to be health promoters – so-called nutraceuticals spanning the boundary between food and medicine – rather than drugs.
It is clear that if we are to target the microbiome successfully we first need to understand the microbial chemistry that plays a vital role in digestion and in sustaining a healthy, fully functioning immune system. Many of the bacteria that are common in our guts produce enzymes called glycosyl hydrolases. These catalyse the hydrolysis of the glycosidic bonds that join simple sugars (monosaccharides) together to form complex carbohydrates. The glycosyl hydrolases are a large, diverse and widespread enzyme family found in organisms from bacteria to humans: common gut microbes contain some with functionality that is not found in the human enzymes. Other enzymes that are unique to bacteria break some of these sugars down further, releasing short chain fatty acids during this fermentation.
Starch, for example, is a complex polysaccharide in which the glucose units are linked together using two different types of linkages, α-1,4 and α-1,6, with the numbers referring to the positions of carbon atoms. It is an essential component of many plant-based foods, but without gut bacteria we would be unable to digest it completely. Resistant starch is not digestible by human amylase enzymes, hence the name. Its digestion even requires synergy between different bacteria: some contain the enzymes that break α-1,4 linkages, and others only break the other linkages. There are other enzymes in the family that nibble single residues from the ends of chains. These exo-hydrolases work symbiotically with the endo-hydrolases that break middle bonds and convert large starch molecules into short oligosaccharide chains, hence increasing the number of ends available. The main end products of all this digestion are the short chain fatty acids propionate, butyrate, succinate and acetate – all with different roles in the body.
Know your oats
The simplest way of improving your microbiome is by altering your diet, and some companies are figuring out ways of providing dietary advice in a precise and even personalised way. Almost everyone in the UK, however, will need to heed the advice to eat more fibre. Diets rich in oats, in particular, are known to correlate with lower cholesterol levels, and Scott’s group has determined one possible reason why. ‘The cell walls of oats and other cereals contain beta-glucans, which travel intact to the human colon where they are broken down by gut microbes into the short chain fatty acids propionate and butyrate. The accompanying reduction in acetate production leads to less cholesterol being synthesised, which in turn helps lower blood pressure,’ she explains.
A pill can help improve your microbiome and lower your cholesterol, but it won’t help much if you otherwise eat nothing but junk
Many people, particularly those with diabetes or on a diet, will have come across the concept of the glycaemic index of a food that describes how quickly it raises blood glucose levels. It is less well known that this response varies between individuals. ‘In some people, glucose levels actually spike more after eating bananas than after eating sweet biscuits, and differences in the microbiome are responsible for this difference’, explains Romanos. DayTwo, a company based in Tel Aviv, Israel, uses this to provide personalised dietary advice to people at risk of obesity and type 2 diabetes. Registered users complete biometric and health questionnaires and submit stool samples; the company analyses their personal microbiomes and uses all the data to generate an algorithm to suggest the foods that will best control their personal blood sugar levels.
Most of the therapeutic agents that have been developed to improve health through directly targeting the gut microbiota are nutraceuticals, regulated less stringently than drugs and which cannot be claimed as medicines to treat disease. There are two main types: food supplements that promote the growth of healthy bacteria, known as prebiotics, and preparations of live, beneficial bacteria, or probiotics.
A purified preparation of the beta glucan found in oats is a good example of a potential prebiotic. Scott believes that these can be very helpful, but mainly for people whose diet is restricted for medical reasons. ‘Eating whole oats is better – if you can’t because you suffer from a bowel disorder, then a pill can help improve your microbiome and lower your cholesterol, but it won’t help much if you otherwise eat nothing but junk’, says Scott. Personalised prebiotics that add back specific products that are missing from the metabolic repertoire of an individual microbiome are likely to be more helpful, but these are still in development.
It is difficult, if not impossible, to measure the active dose of a probiotic
The term ‘probiotic’ may refer to any product that contains live bacteria and that has health-promoting properties, from ordinary ‘live’ yoghurts to purified preparations of bacteria. These contain bacteria from genera and species found in the healthy gut, including lactobacilli and bifidobacteria. ‘These bacteria, found in the guts of infants and children, may help strengthen adult immune responses too’, explains Eamonn Quigley, a gastroenterologist based at Houston Methodist Hospital in the US. There is a developing interest in the idea of combining probiotics and prebiotics into so-called synbiotics; a recent study in India has shown that a mixture of the probiotic Lactobacillus plantarum and a fructo-oligosaccharide prebiotic can reduce the incidence of sepsis, lower respiratory tract infections and death in young infants in a poor rural population.
Many questions remain about the use of probiotics, not least how they can be tested. ‘It is a quality control issue,’ explains Quigley. ‘It is difficult, if not impossible, to measure the active dose of a probiotic, as this will depend on the proportion of live bacteria that survive their journey down the GI tract and reach the colon unscathed.’
The yuck factor
Delivery of live bacteria to the colon of a patient with a disordered microbiome does not have to start at the mouth, however. Faecal microbial transplantation (FMT), which involves the transfer of liquefied stool from a healthy donor into a recipient patient, is regularly used for the treatment of recurrent diarrhoea caused by Clostridium difficile infection, and it is being investigated for other indications. The Quadram Institute in Norwich, UK, has one of only three FMT facilities in the country that can provide samples for use in the NHS. ‘Stool samples are delivered straight into the small intestine through a tube inserted into the nose’, explains Simon Carding, a senior researcher at the Quadram Institute. ‘The only side effects are mild, transient nausea and bloating, and, perhaps surprisingly, our patients like it: there is very little of the “yuck factor”.’
Carding’s group is exploring the use of FMT in other diseases, including neurodegenerative disease and chronic fatigue. The Motion study involves tracking the gut microbiome and cognitive function over time in initially healthy adults from the age of 65, looking for changes in the microbe population that correlate with cognitive decline. Faecal transplantation from young mice to aged ones has been shown to increase their cognition, and this is being tested in a more relevant primate model of neurodegeneration. ‘If this treatment shows success in our macaques, we will move to clinical trials, initially for Parkinson’s disease,’ adds Carding. The group is also investigating whether stool transplants from healthy donors can be effective in treating the fatigue and ‘brain fog’ that are characteristic of myalgic encephalomyelitis or chronic fatigue syndrome.
Drug the bugs
It is possible to target the microbiome with small-molecule drugs, however, if it is done indirectly with compounds that inhibit the enzymes found in gut bacteria. Emily Balskus, a chemical biologist at Harvard University in the US state of Massachusetts aims to develop drugs that do just that. ‘We are not developing antibiotics that kill microbes or inhibit their growth, but compounds that modulate some of their specific metabolic functions,’ she explains.
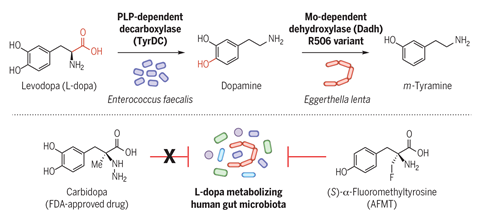
The treatment of Parkinson’s disease is complicated by a wide variation in patients’ initial response to the most important drug used for this condition, l -dopa. We now suspect that much of this disparity could arise from a difference in the extent to which l -dopa is inactivated in the colon by decarboxylase enzymes produced by gut bacteria. ‘We searched gut microbiome databases for likely enzymes and found a tyrosine decarboxylase in Enterococcus faecalis that decarboxylates l -dopa as well as its preferred amino acid substrate,’ says Balskus. Specific inhibitors of this enzyme will target this unwanted activity while sparing the other beneficial functions of this common gut microbe; the first inhibitors developed through this programme are being tested in animal models.
Trimethylaminuria is a rare metabolic disorder in which the oxidation of trimethylamine (TMA), a metabolite of choline, is blocked, leading to a build-up of the amine. People suffering from this disorder excrete the excess TMA, which has a characteristic, unpleasant fishy smell. Balskus’ group is developing cyclic choline analogues as inhibitors of the bacterial enzyme choline-TMA lyase; this will block the pathway earlier by inhibiting the production of TMA, and so prevent the build-up of the malodorous metabolite. Balskus is hopeful that these efforts might lead to the development of drugs for rare and degenerative diseases.
Adding drugs that inhibit bacterial enzyme action to the various prebiotics, probiotics, synbiotics and FMT applications in the clinic is likely to extend even further the range of conditions that can be helped by targeting the gut microbiome. In future, we should be able to adapt these treatments to the precise nature of each individual’s microbiome, as DayTwo is doing with its straightforward dietary advice. The tiny friends in our guts will treat us well throughout our lives but only if we – now, with the help of the pharma and biotech industry – repay the compliment.
Clare Sansom is a science writer based in Cambridge, UK













No comments yet