Chemistry World recently spoke to Ryo Horikoshi, a chemist at Okayama University of Science, about the stunning paper models of supramolecular structures he has been crafting for his undergraduate students. He told us about how he came up with the idea, how he wants to inspire students to love supramolecular chemistry as much as he does and how the models are simple enough to create that anyone can make them with a little time and patience.
Readers keen to make these beautiful models themselves can watch Ryo Horikoshi make one and find the templates that he has used to craft these papercraft supramolecular wonders.
What made you turn to papercraft to convey chemistry concepts?
I have been a member of Okayama University of Science since 2024, where I teach basic chemistry and labs to mainly first-year students in the department of biology and geology. Some of them are good at chemistry, others are not so good.

Why did I start using papercraft teaching materials? I heard that Professor Makoto Fujita at the University of Tokyo, a leading researcher on metal–organic cages/cycles (MOCs) and crystalline sponges, had made his laboratory paperless. I had wanted to introduce MOCs to students for long time and it occurred to me that it might be possible to create papercraft models of Fujita’s MOCs and crystalline sponges. It’s quite possible that traditional Japanese origami may have inspired me.
I’ve always been very playful in my teaching
Ryo Horikoshi, Okayama University of Science
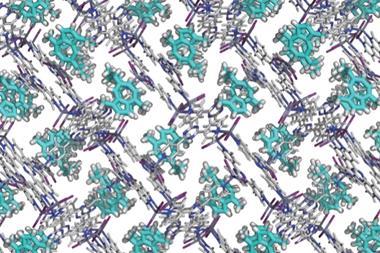
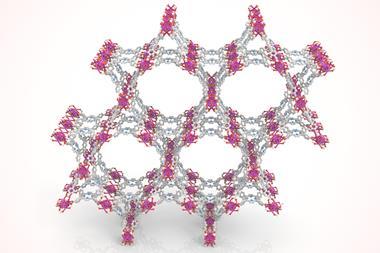
MOCs comprise metal ions and organic linkers, with coordination bonds between them, represented by paper clips in the papercraft models. As MOCs and metal–organic frameworks (MOFs) are beautiful structures, my lectures at university and high schools, as well as scientific events, that feature these papercraft models have become very popular.

Why do you focus on these structures?

Isn’t the structure of MOCs and MOFs very architectural complex and beautiful? I have always wanted to convey this beauty to students. I like metal complexes because I was in a coordination chemistry lab as a postgraduate, and I have had research published in Dalton Transactions and the New Journal of Chemistry. My colleagues and I recently submitted our manuscript to CrystEngComm and it was accepted.
First-year university students are not used to looking at skeletal structures or crystal structure diagrams, so I thought it would be useful to have models that they could hold in their hands and learn from. MOCs and MOFs are also excellent topics to learn about because they are compounds whose properties are easy to understand from their structures. A large hollow compound that encapsulates smaller compounds can be imagined by even beginner chemistry students.
Have you produced papercraft structures of other supramolecular structures?
Although not supramolecules, I have created papercraft models for crown compounds and sandwich complexes. Lectures using the former will start in 2025 and the latter in 2026 and I plan to report back on how these are received in the Journal of Chemical Education.
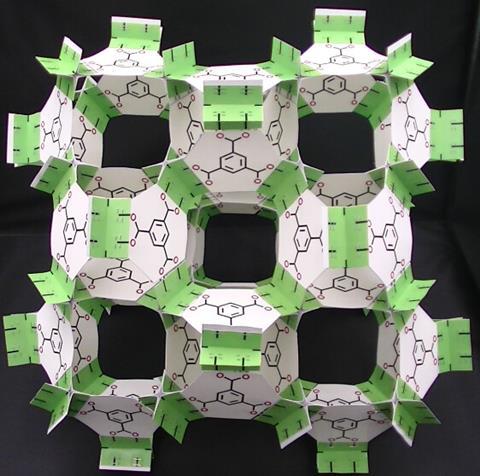
Which chemistry Nobel prizes have featured in your models?
As I said, I plan to use papercraft models to explain the structures and properties of crown compounds, which won the 1987 chemistry Nobel prize, and sandwich complexes, which won the 1973 prize. Personally, I expect that MOCs or MOFs or both will soon receive the Nobel prize in chemistry.
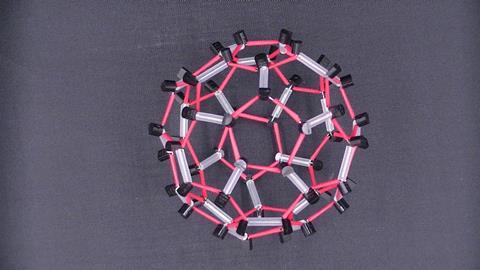
I’ve also used models made out of electronic components to explain the organic conductor TTF-TCNQ, with a brief mention of the 2000 Nobel prize in chemistry, and C60, which won the 1996 chemistry Nobel prize. I’ve always been very playful in my teaching and wanted to make electronic materials, such as C60, nanotubes, TTF and TCNQ, out of electronic components such as transistors and LEDs. I enjoy the inverted concept of crafting electronic materials from electronic components.
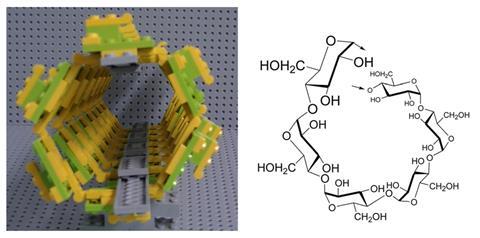
I’ve also taught asymmetric hydrogenation catalysis (2001 chemistry Nobel prize), olefin metathesis reactions (2005 chemistry Nobel) and cross-coupling reactions (2010 chemistry Nobel) with Lego. In his 2010 Nobel prize in chemistry lecture, Professor Ei-ichi Negishi compared catalysis to playing with Lego bricks – after reading this I decided to give it a go.
How many different models have you made so far?
I’ve created around 10 papercraft MOCs and five papercraft MOFs so far and will be teaching lectures with some of them this year. All in all, I have developed about five molecular models made from electronic components and five from Lego. It’s all about making studying chemistry fun.
How long does it take to design and create these models?
The design of Professor Fujita’s MOCs papercraft models took about a month of repeated trial and error. At first, the coordination bonds were represented by neodymium magnets, but as magnets are expensive and the model could not withstand their weight, I moved to paper clips.
In total, the papercraft model of the MOF UiO-66 took about eight hours to build. I worked on it little by little, with the help of a beer in the middle of the night!
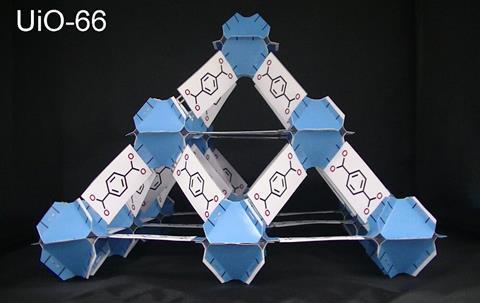
Can anyone make these models?
Papercraft MOCs are so simple to make they can be built by pre-school children with the help of an adult. MOFs, on the other hand, are easy to construct but time-consuming.
The papercraft models don’t need special materials, they are made using card printed with molecular structures. Tools needed include scissors, stapler, sticky tape and (for adults) beer.
Would you recommend using papercraft models to teach chemistry to others?
I would recommend papercraft models to everyone because the materials are inexpensive and easy to craft, and the finished models can be seen and held. However, the longer the crafting takes, the less time there is for lectures, so the instructor needs to have done the prep work. I have included instructions in my papers for other teachers to follow to make these beautiful structures.
Have students found these papercraft models helpful?
Post-lecture questionnaires showed that many students enjoyed these lectures. Perhaps I should test the students after one of these lectures to estimate the pedagogical impact of the models. However, I don’t want to spoil their enjoyment with an exam, so I won’t do that!
This interview has been edited for brevity and clarity













No comments yet