Chris Nawrat
Chris Nawrat obtained his PhD in total synthesis in the UK, completing several brightly coloured natural products. After a postdoctoral stint at an Ivy League school, he remained in the US, where he now works as a process chemist at a major pharmaceutical company.
 Opinion
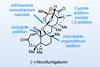
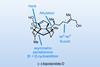
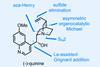
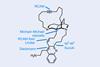
Opinion(−)-Novofumigatonin
Oxidations abound in this satisfying synthesis, with a delicate nitrile hydrolysis to finish
 Opinion
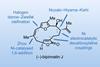
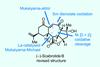
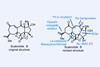
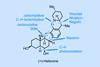
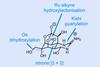
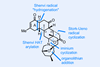
Opinion(–)-Scabrolide B (again!)
Proverbially, comparison may not bring joy – but it can be educational
 Opinion
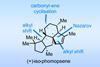
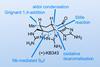
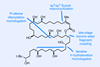
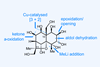
Opinion(+)-iso-Phomopsene (and friends)
A non-obvious combination unleashes the power of electrocyclisation
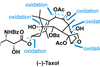
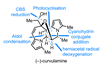
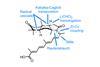 Opinion
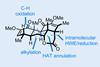
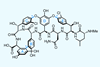
Opinion(±)-Rhabdastrellic acid A
A triterpene that looks innocuous until it reveals the highly strained conformation of its rings
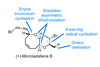 Opinion
Opinion(+)-Microladallene B
A weird structure is enough to spark chemists’ interest, even without any obvious use