A short route that brings together the classical and cutting-edge
I’ve never tried ChatGPT. I’m not sure if this will be surprising to the readership of this column, but it certainly makes me a standout among my coworkers who use it constantly for anything from checking grammar and changing the tone of emails to writing rap battles between drugs and composing haikus on process development. Now I’m no luddite, and I’m not against the technology per se, but I’m not quite sure where it adds value or would fit into my day-to-day. Unlike most of my co-workers, I also enjoy the process of writing.
I’m sure this gap between invention and adoption can be seen in many fields, and it’s certainly present in organic chemistry. Even the most widely-read and knowledgeable bench chemists can be leery of new reactions and technologies – and I am as well. The thing is, even if we believe they’ll work, is it really worth the trouble of buying new catalysts or equipment? It’s hard to keep on top of the pros and cons of all the new methods being published, so why not stick with the safe route, even if it’s a step or two longer?
One area where I think the battle for widespread adoption has just about been won is photoredox chemistry; most chemists I work with won’t think twice about grabbing a once-exotic iridium catalyst and some LEDs if they think it’ll save them some time. This would have been unimaginable to me even a decade ago when I was finishing my PhD and photochemistry felt more like a fetish than a field. The battle for electrochemistry continues.
One once-hyped technology that I haven’t seen widely adopted in complex molecule synthesis, particularly in industry, is C–H functionalisation. Yes, in principle it’s cool to just use those ubiquitous C–H bonds directly without having to go via some other intermediate, but the reality is often less appealing. Poor selectivity in the absence of directing groups, very high catalyst loadings and mediocre yields are all still relatively commonplace. But, when it fits the task at hand, there’s no doubt that C–H functionalisation can be a time-saving strategy, as the recent synthesis of (+)-heilonine by Mingji Dai and coworkers at Emory University in the US demonstrates.1
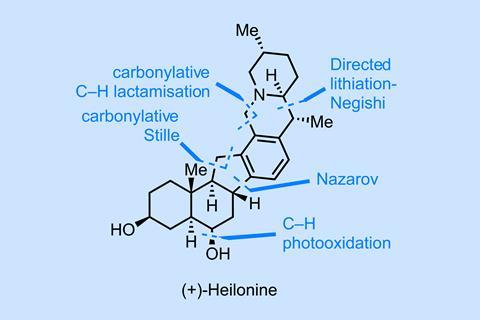
The route begins with one of the oldest C–H functionalisations – directed metallation. Here, the conformation of the piperidine ring (driven by the existing stereogenic centre) leads to a highly diastereoselective equatorial lithiation (figure 1). Transmetallation and Negishi coupling then prove to be an efficient way to attach the D-ring. Deprotection followed by substrate-directed hydrogenation of the exo-methylene with Crabtree’s catalyst then sets the methyl stereogenic centre. From here, a neat little carbonylative lactamisation and a rhodium-catalysed iodination wrap up the tricycle synthesis with some true metal-catalysed C–H activation magic – all at reasonable loadings.
Jumping ahead a little, the final ring is closed via Nazarov cyclisation (figure 2), which again functionalises a seemingly inert position on the aromatic D ring for the third time in the synthesis. (If the only total syntheses you read are those in this column, you probably have a vastly inflated sense of how common this reaction is – no one I work with has ever run one.) The reaction does favour the desired diastereomer but the selectivity isn’t stellar; fortunately, the unwanted isomer can be epimerised and recycled. Removal of the unwanted carbonyl is also unexpectedly tough and can be done either directly via a Clemmenson reduction or a longer – but higher-yielding – Barton–McCombie sequence.
With a longest linear sequence of just 11 chemical steps, this is an impressively short synthesis of a complex steroidal alkaloid and the group does a great job of marrying classical organic chemistry with some cutting-edge catalytic methods. A perfect example of this combination of old and new is the photocatalytic oxidation of the Wieland–Miescher ketone the team develops to shorten the route – but you’ll have to go and read the paper to see that.
References
1 Y Jin et al, J. Am. Chem. Soc. 2024, 146, 1825 (DOI: 10.1021/jacs.3c13492)














No comments yet