A stepping stone to greater things?
During a recent career panel for college students, I was asked when I knew that I wanted to do a PhD in organic chemistry. Of course, as is often the case, I don’t think there was a single Damascene moment when I suddenly found my calling. But I do remember the first time I found organic chemistry fascinating, which was in Gerry Pattenden’s third year undergraduate course on natural products, which I believe was titled ‘Medicines from Nature’. As Gerry recounted stories about these powerful and beautiful molecules and the legendary scientists who jockeyed to understand and eventually make them, I was hooked.
One of the things that most intrigued me was learning that the majority of natural products don’t exist in isolation. For example, while morphine might be the most famous of the benzylisoquinoline alkaloids that are produced by the opium poppy, that species alone makes over 100 closely related molecules in the same family. Putting them side by side, it’s possible to speculate about the relationships between them, look for common ancestors and then start to guess about the chemistry and enzymes that nature might use to assemble and convert between them. This sort of biosynthesis-inspired thinking has led to some of the most elegant total syntheses ever reported, including many of my favourites.
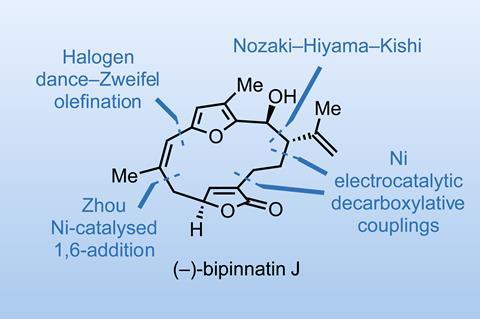
Recently, Phil Baran and coworkers at Scripps Research in California, US, reported a neat total synthesis of bipinnatin J.1 We’ll discuss the route in a moment, but first, let’s ask why the group is working on this natural product.
It’s actually a bit of an odd target, as several good routes have already been reported. Sure, Baran’s is shorter and more efficient than the previous ones, but I suspect the molecule is just a stepping stone to some of the more complex, biosynthetically related molecules in this class, some of which have remained elusive despite significant effort by the community. Much like how the group’s early work with simple taxadienes eventually culminated in the conversion of these into Taxol itself. So watch this space!
Back to the synthesis and the preparation of the northern furan fragment. After several unsuccessful approaches to make a key Z-bromoalkene, the team figured out an unlikely sequence (figure 1). After deprotonation and ’halogen dance’ rearrangement, the lithiated furan is treated with an alkenyl boronic ester to give a boronate intermediate. Addition of N-bromosuccinimide (NBS) then effects a 1,2-shift and anti elimination to add a vinyl group to the furan with complete inversion of the original vinyl boronic ester geometry. Surprisingly, the vinyl trimethylsilyl (TMS) group can later be swapped to a bromide without much scrambling of the double bond.
Work towards the southern half of the molecule begins with a glycidol-derived butenolide, which is cross-coupled to an alkenyl side chain using the group’s signature electrocatalytic decarboxylative methodology. This reaction works reasonably well, but the next steps don’t go according to plan. The group had intended to activate the tert-butyldimethylsilyl (TBS)-protected alcohol and cross-couple again with the furan-containing vinyl bromide fragment. Unfortunately, any activation resulted in an elimination reaction and the loss of the molecule’s chiral information.
The group’s bold solution was to embrace the elimination and instead restore this stereocentre later (figure 2). Selective nickel-catalysed 1,6-addition couples on the vinyl bromide, and an asymmetric proton transfer using Deng’s cinchona catalyst then restores the lost stereocentre with surprising efficiency, putting the target within touching distance.
There has been much speculation about how bipinnatin J could be related to other, much more complex marine natural products such as bielschowskysin, and I’m fascinated to see where the group goes next.
Full disclosure: The author spent an eye-opening, though largely unsuccessful, stint in the Pattenden lab during his master’s year, where he got his first taste of natural products research.
References
1 A J Rodriguez et al, J. Am. Chem. Soc., 2025, 147, 16781 (DOI: 10.1021/jacs.5c04761)













No comments yet