Rods, stars or spheres? Rachel Brazil looks at the shape of things to come
Size matters – that’s the basic foundation of nanotechnology. Below 100nm, quantum mechanical effects start to dominate and the properties of materials are affected by particle size: a 10nm gold nanoparticle won’t necessarily behave the same as a 100nm version. But what about shape? Does it matter if a particle is spherical, rod-like or star-shaped? As nanotechnology moves towards applications in electronics and medicine, it’s becoming clear that shape plays an equally important role.
The classic illustration of ‘size matters’ in nanotechnology is a series of colloidal gold suspensions, containing different sizes of gold nanospheres. As particle size increases, the colour of each solution changes from a rich red through pink to pale purple. This is because of surface plasmon resonance, the oscillation of free or conducting electrons at the particle surface. As particle size increases, the absorbed wavelength shifts, creating a bluer solution.
What this neat example usually leaves out is what happens when the nanoparticles are not spherical. For example, gold ‘nanourchins’ – spiky sea-urchin shaped particles – have very different optical properties. Colloidal suspensions of 100nm gold nanourchins are blue. Their uneven surface and spikes cause large electromagnetic field enhancements, leading to a red-shifted surface plasmon absorption compared with that of equivalently sized spheres.
Colour is not the only property affected by shape. In the case of energy storage materials, Jennifer Dionne of the Stanford Institute for Materials and Energy Science in the US has been looking at optimising palladium nanoparticles for hydrogen storage and release to model the potential processes occurring in the charging and discharging of lithium-ion batteries. Nanomaterials could be ideal for battery materials as they are likely to withstand cycling with less structural changes.
Shaping properties
Dionne recently found that 50nm nanocubes and pyramids were able to store more hydrogen than the icosahedral particles.1 She attributes the difference to the crystal structures that make up the three shapes. The cubes and pyramids were single crystals, which allowed uniform hydrogen absorption – while the icosahedra were multiply twinned particles containing internal compressive strain and structural defects that the hydrogen was unable to fully penetrate. ‘You can think about the icosahedral nanoparticle a little bit like the Earth – there is a lot of pressure at the centre and that prevents hydrogen from getting in there,’ says Dionne.
‘We have ongoing results that imply that shape is also important in terms of the kinetics – the speed with which you are cycling your energy storage material,’ she says. ‘There are things like the surface faceting – which surfaces are exposed on the nanoparticle – that also seem to be playing a significant role.’
Shape is imporant in terms of the kinetics of energy storage
Chemist Teri Odom from Northwestern University near Chicago, US, has looked at how nanoparticle shape can improve the performance of contrast agents used for magnetic resonance imaging (MRI). Image contrast in MRI is affected by the relaxivity of protons in water molecules and this itself is affected by the type of biological tissue the water molecules are in, as well as a characteristic time constant known as the relaxation time. Commercial contrast agents are usually gadolinium(III) ions, chelated to macrocyclic molecules. The gadolinium coordinates water molecules, creating dipolar interactions with the ion’s seven unpaired electrons. This shortens the nearby water molecules’ relaxation time, increasing image contrast.
Odom, together with Northwestern colleague Thomas Meade have looked at a new class of gold nanoconjugates. They covered 40nm gold particles with complexed gadolinium(III) ions conjugated to thiolated DNA molecules with 24 nucleotides, but decided to look at the effect of using nanostars as well as nanospheres. They found that stars provided the highest relaxivity results – three times higher than the comparable spheres and up to 25 times higher than current agents.2
Odom says they worked out this was due to the environments created for coordinated water molecules. In standard gadolinium contrast agents, one water molecule is transiently bonded to the metallic centre at close range (0.25nm) – known as the inner sphere. But with nanostars something else was happening. The star shape has concave areas on its surface and this affects the organisation of the conjugated DNA and creates dense hydrophilic microenvironments in the proximity of the gadolinium complex.
‘Our hypothesis was that the gadolinium atoms in the interstitial curvature regions are able to interact with more than just one water molecule per gadolinium,’ says Odom. ‘They were able to actually interact with a second shell of water.’ Known as outer-sphere relaxivity, the powerful paramagnetism of the gadolinium ion is able to transiently interact with a second water shell about 0.4–0.5nm away, leading to much higher relaxivity than that seen with the spherical nanoparticles.
Shaping nanomedicine
There are already 16 approved nano-drugs and many more in development. Understanding the effects of shape is crucial for more successful nano-medicines. For over a decade, Samir Mitragotri at the University of California, Santa Barbara in the US has been investigating how nanoparticles are transported around the body and what role size and shape plays. Size is certainly important. Particles larger than 10nm will get immediately scooped up by the body’s immune system and taken to the liver for destruction and removal.
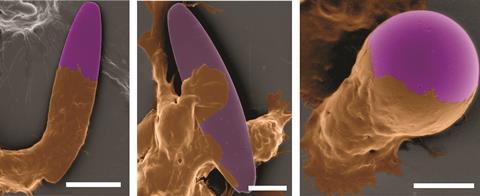
But understanding how best to deliver nanoparticle drugs and fool the body’s immune systems is complex. ‘How do they target their cells, how do they get internalised – all these features, all these outcomes are impacted by the nanoparticle shape,’ says Mitragotri. One trick is to use nanoparticles that stick to cells, which can then transport them around the body. Mitragotri has found that rod-shaped nanoparticles adhere better than spherical particles – probably because elongated nanorods have a larger surface area in contact with the cell.
The other difference between nanorods and spheres is their likelihood of being internalised by a macrophage – the white blood cells responsible for digesting foreign material in the blood stream. Spheres seem to be engulfed more easily than rods or discs. Mitragotri explains that the process is connected to the polymerisation of the protein actin under the cell membrane, which generates a force that drives the cell membrane around the nanoparticle. ‘If a rod- or disc-like particle binds to the macrophage, the actin polymerises all over the place but it’s not coordinated,’ he says. ‘So you get blobs of polymerisation but not the formation of a well-defined ring that is necessary to uniformly engulf the particle.’
The phenomenon was tested by Mitragotri injecting mice with polystyrene nanorods and spheres of the same volume – 200nm diameter spheres and rods 500nm long by 120nm wide. The nanoparticles were coated with antibodies that attach them to endothelium cells, which line the inside of blood vessels. He found twice as many nanorods as nanospheres in the animals’ lung tissue and accumulating in brain endothelium cells.3 So nanoparticle shape could also provide a route to targeted drug delivery.
Mitragotri is also targeting inflammation with what he calls ‘cellular backpacks’. He has assembled large flat disc-like shaped polymeric nanoparticles, 200nm thick and 7μm in diameter, designed to piggyback on monocytes – a type of white blood cell that migrates to inflamed tissue as part of the body’s immune response. The shape is key to their success, Mitragotri explains. ‘Because they are large and flexible, the monocyte is not able to internalise them, and because again they are so thin, the monocytes can carry them in the circulation and still escape the blood vessels to go to the tissue without shedding the backpacks.’
The backpacks are made from layers of poly(methylmethacrylic acid) and poly(allylamine hydrochloride) (PMMA and PAH), as well as 10nm iron oxide nanoparticles for strength, and covered in antibodies on one side to stick to monocytes. So far he has shown in mice that his backpacks can in principle target inflammation and in future, the idea is to add therapeutic agents to the backpacks.
Mitragotri has also designed artificial platelets with a distinct shape. Platelets are the disc-shaped cell fragment, found in blood, which promote clotting at injured vascular sites and their shape is key to their function. Mitragotri’s team created platelet-like nanoparticles (PLNs) that are flat and flexible and exhibited enhanced surface-binding compared to spherical or rigid discoidal counterparts. ‘We wanted to make sure that they are not only similar in shape, but also soft and flexible just like natural platelets,’ says Mitragotri. In blood flow, the flexible disc-like particles tend to be forced to blood vessel walls where they can aggregate.
The 200nm PLNs are synthesised around spherical polystyrene nanoparticles which are coated with four bilayers of PAH and the protein bovine serum albumin. The polystyrene core is then dissolved leaving a flexible disc. Coating with various peptides also assists collagen binding and other pathways that attract platelets to aggregate. Mitragotri tested the artificial platelets using standard bleeding experiments in mice – which involve ‘tail transection’ – cutting off 1–5mm from the tip. These showed PLNs could take 35% off clotting time.
Other drug delivery strategies are using inorganic nanoparticles: for example, Odom is seeing if 25nm gold nanostars can deliver DNA drugs to cancer cells. They key to all these nanoparticle delivery strategies is their ability to target the right cells. This is particularly crucial in cancer therapies where drugs are often toxic. In April, a meta-study of 117 papers on nanoparticle delivery to tumours, by chemist Warren Chan at the University of Toronto in Canada, found that only an average of 0.7% injected nanoparticles reached their target.4 More worryingly, that figure had been constant over the last decade. Perhaps a closer look at shape may provide some answers? ‘We can do better, I guess that’s why we are trying to engineer these things at the nanoscale,’ says Odom.
Growing bone with nanoparticles
Nanoparticles in medicine go beyond drug delivery though, having interesting effects on stem cell development: gold nanoparticles can promote the differentiation of stem cells – but when surface-modified, they can also inhibit it. But what is the effect of their shape? The group of Guoping Chen at the National Institute for Materials Science in Tsukuba, Japan recently looked at precisely this.5
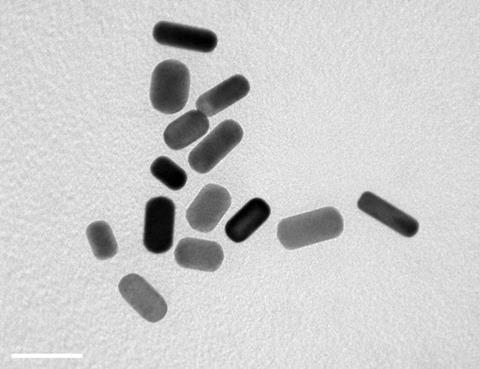
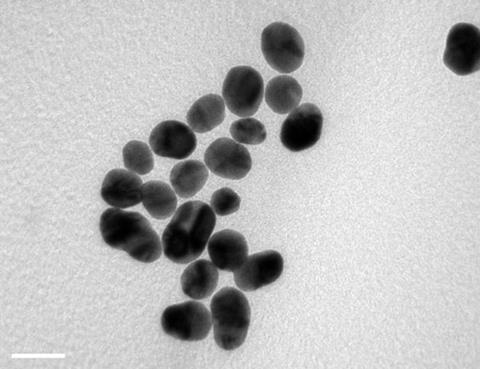
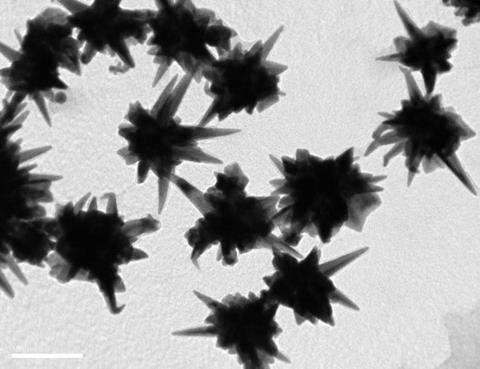
They investigated the effect of a variety of shapes and sizes of gold nanoparticles on the differentiation of stem cells into bone cells. Three sizes of gold spheres, rods and stars were synthesised by citrate reduction of chloroauric acid (HAuCl4) solution onto very small 15–20nm gold ‘seeds’, creating the different sized particles by varying reactant concentrations. Rods were formed by adding the surfactant cetyltrimethylammonium bromide which ‘templates’ the growth of rods. Nanostars were formed by adding silver nitrate. The silver ions inhibit uniform growth on the gold seed surface, leading to star formation.
Chen and colleagues found that cell development was affected by the shape and size of nanoparticles internalised by the cells. Through looking at the expression of marker genes for bone growth and the amount of calcium deposited after three weeks, they showed that while most of the nanoparticles catalysed the differentiation of stem cells to bone cells, only the 40nm rods retarded the process.
The result is still a puzzle for Chen’s colleague Jingchao Li, who worked on the project. ‘Many papers report that the nanoparticles inside the cells may cause mechanical stress. We have found that the different size and shape [of nanoparticles] will cause different influences on this stress, but we cannot confirm how and why this influence exists,’ he says. The suggestion is that once inside stem cells, nanoparticles can cause activation of some signalling pathways through mechanical or electrochemical effects, but it is not yet clear why shape should play a role although it seems to do so.
Li says the result could be useful to provide a route for treating some bone diseases where there is an imbalance of cell breakdown and restoration. ‘Bone resorption is necessary to maintain homeostasis for bone remodeling, but excessive resorption leads to osteoporosis. Excessive bone resorption is mostly caused by increased osteoclast [bone cell] formation. So a nanoparticle to inhibit this could be good for our health,’ he says.
Making shapes
Given its importance, it’s not surprising that chemists have been coming up with new methods for producing almost any shape of nanoparticle, particularly for drug delivery systems. Liquidia, a start-up from the University of North Carolina lab of Joseph DeSimone in the US is able to manufacture particles of controlled size, shape and composition using his Print (particle replication in nonwetting templates) templating process. In 2015, GSK acquired the rights to use the method to manufacture inhaled particulate drugs.
The technology, which borrows lithography principles from the semiconductor industry, uses photochemically cross-linked fluoropolymer templates with nanoscale cavities. Drug precursor solutions added to the mould solidify into nanoparticles which are then transferred onto a ‘harvesting film’. This is then dissolved to yield free-flowing drug particles in shapes from cubes to doughnuts or boomerangs.
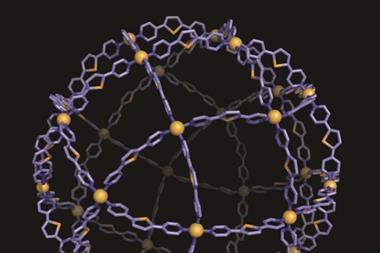
Another ingenious method for creating sub-25nm particles of any shape uses DNA – or more specifically DNA origami. First reported in 2006 by Paul Rothemund from the California Institute for Technology in the US, the process uses single-stranded viral DNA (around 7000 base-pairs) ‘stapled’ or folded into rigid shapes by complementary short DNA strands – the staples. Through computer-aided design, the DNA can be folded into almost any shape.
In 2014, Peng Yin from Harvard and Mark Bathe from MIT in the US showed how this method could be extended to construct 3D DNA moulds to produce metal nanoparticles.6 As Wei Sun from Yin’s Harvard group explains, ‘Arbitrarily prescribed shapes – both symmetric and asymmetric – can be rationally designed and produced through our strategy. This is similar to the remarkable ability of nature to encode diverse three-dimensional shapes and target functions of proteins into the linear genome.’ Once the moulds have been designed, the DNA elements are thrown together and incubated for 2–3 days to allow self-assembly. Gold nanoparticle seeds are attached to the moulds via complementary DNA ‘handles’ and allowed to grow in chemical solution, filling up the available space provided by the mould. Once formed, the outer DNA layer can be dissolved or even kept to add additional surface functionality.
Using this method, Sun says they have created nanoparticles in silver and gold: spheres, Y-shapes, and more complex nanoparticles like a cube sandwiched between two spheres. The precision is high, with a tolerance around 5nm – better than most printing or solution-based templating methods can offer – although sometimes, as with any casting process, they have problems with sharp corners that tend to get rounded off.
The Yin group, in collaboration with IBM, is now looking at how to create nanoelectronic or photonic circuits and components – with DNA effectively providing the assembly. ‘We are trying to nucleate semiconducting materials to see if these nanomaterials can be grown,’ says Sun.
With these increasingly sophisticated nano-fabrication technologies making almost any type of particle, the challenge for nanotechnology is to find the right shape for the job – be it spherical, discoid or nanostar. Because while size has always mattered, we are now learning that shape matters too.
Rachel Brazil is a science writer based in London, UK
Article amended 16 August to correct Wei Sun’s name.
References
1 T C Narayan et al, Nat. Mater., 2016, 15, 768 (DOI: 10.1038/nmat4620)
2 M W Rotz et al, ACS Nano, 2015, 9, 3385 (DOI: 10.1021/nn5070953)
3 P Kolhar et al, Proc. Natl. Acad. Sci. USA, 2013, 110, 10753 (DOI: 10.1073/pnas.1308345110)
4 S Wilhelm et al, Nat. Rev. Mater., 2016, 1, 16014 (DOI: 10.1038/natrevmats.2016.14)
5 J Li et al, Nanoscale, 2016, 8, 7992 (DOI: 10.1039/c5nr08808a)
6 W Sun et al, Science, 2014, 346, 1258361 (DOI: 10.1126/science.1258361)










No comments yet