Nina Notman reports on progress towards products that can, when necessary, replace donor red blood cells
A 23-year-old US male arrives in hospital in severe hemorrhagic shock after being crushed between two cars. A critical part of his treatment would normally be to administer donated blood, to replace the volume lost due to the accident. But this young patient is a Jehovah’s Witness, a Christian denomination whose members refuse blood transfusions. His heart rate, blood pressure and the levels of haemoglobin, and consequently the amount of oxygen, in his blood are all dangerously low. Conventional treatment alternatives aren’t helping. Thankfully his doctors were aware of an alternative – though unapproved – approach, and 30 hours after his accident the patient is administered with the haemoglobin-based oxygen carrier (HBOC) Hemopure. HBOCs mimic the behaviour of the natural protein haemoglobin in red blood cells, transporting oxygen around the body, and are often called blood substitutes or artificial blood. The patient quickly rallies, and is discharged from hospital less than two weeks later.1
No HBOCs are currently approved for use on humans in the US or in Europe. Hemopure was administered to this patient through the US Food and Drug Administration’s (FDA) expanded access programme. This programme, often referred to as compassionate use, allows people in serious or life-threatening conditions access to certain unapproved products. And while this patient was lucky that his clinicians quickly thought outside the box, he was not uniquely so. ‘The product is currently used on a weekly basis in the US in patients for whom blood is not an option,’ explains Zafiris Zafirelis, president of the Pennsylvania-based company HbO2 Therapeutics that produces Hemopure. ‘We’ve treated, so far, around 2000 human subjects worldwide.’
A chequered history
The aim isn’t to wholesale replace donated blood with an artificial product, but to treat a number of patient groups – including those who can’t receive it for religious reasons – for whom an alternative is needed. Patients who receive regular blood transfusions for conditions such as sickle cell disease, for example, can develop life-threatening immune responses to donated blood. And rare blood types can be difficult to source.
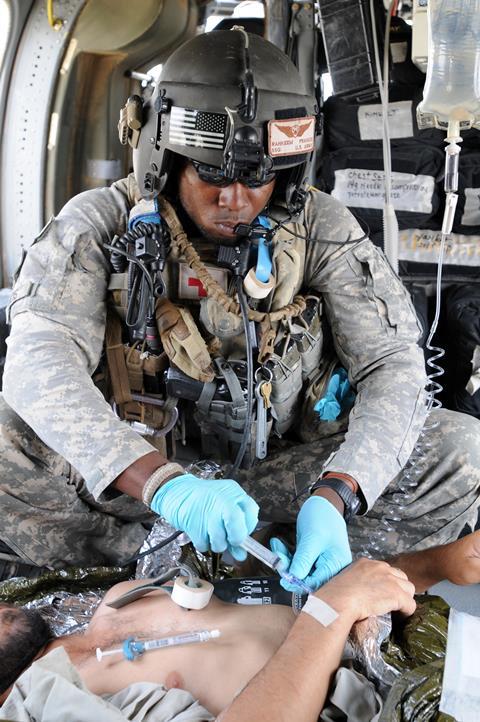
There is also the practicality issue: HBOCs are universally compatible, so blood types don’t need to be checked before use. Their storage is also often appealingly practical; for example, Hemopure remains stable at room temperature for at least three years, whereas red cells from donor blood can be refrigerated for 42 days maximum. It’s no wonder that the US military has funded a significant amount of the HBOC research to date. ‘These products can sit in a helicopter, ambulance or a backpack of a medic for years without spoiling,’ explains Jonathan Jahr, an anaesthetist at the University of California, Los Angeles, school of medicine, who advises a handful of companies developing HBOCs. ‘They can be infused rapidly into someone who’s bleeding to death to resuscitate them enough to get to a higher level of care, dramatically increasing their chances of survival.’
HBOCs also circumvent concerns about donated blood contamination, which began in 1980 with the media spotlight on HIV. South Africa – believed to have more people living with HIV than anywhere else – is one of the few countries to have approved an artificial blood product; Hemopure in 2001.
With still an obvious clinical need, why have no HBOCs been approved by the US or EU regulatory bodies? In part this is due to poorly designed clinical trials with the early blood substitutes, explains Jahr. ‘This has happened many times in this domain.’ Then, in 2008, the field received a near fatal blow: the publication of a meta-analysis of findings for 16 clinical trials of five different products. The paper concluded that HBOCs significantly increase the risk of death and heart attack.2
Many letters to the editors outlined the community’s dismay at the unfairness of lumping all the products together; a 2015 reassessment by Jahr and colleagues confirmed that eliminating one long-abandoned product from the meta-analysis would have reversed its conclusion.3 But the damage was done. The FDA became extremely cautious, and funding started to dry up. Polyheme’s Northfield Laboratories and Hemospan’s Sangart are two HBOC developers that have since gone bust. Hemopure nearly suffered a similar fate. Its developer Biopure filed for bankruptcy in 2009. The buyer, OPK Biotech, went bankrupt itself in 2014, and was bought by the newly formed HbO2 Therapeutics.
Jahr does, however, see light at the end of the tunnel: ‘I see a slight change in the FDA’s stance and I’m hoping the EU [regulatory body] as well.’ They are starting to better judge the risk-to-benefit ratio of these products, he believes. ‘Efficacy has never been the issue for these products, it’s always [often unfairly] been about safety.’
And it’s not the case that the US and EU have never approved a blood replacement. Fluosol, a perfluorocarbon-based oxygen carrier, was approved by the FDA in 1989 but withdrawn again in 1994, due to complexity of use and side effects. Then in 1998, Hemopure’s veterinary equivalent Oxyglobin was approved to treat canine anaemia in the US and Europe. ‘More than 175,000 animals of various species have now been treated already really successfully with Oxyglobin,’ says HbO2 Therapeutics’ Zafirelis.
Keep it fresh
Jahr foresees an HBOC being approved for use in humans in one niche area first, and this then opening doors for funding and further approvals for other applications. HbO2 Therapeutics is currently pursuing Hemopure as an organ perfusate, a fluid for storing harvested organs before they are transplanted.

HBOCs contain natural non-human haemoglobin, normally sourced from cows. ‘Hemopure is a polymerised bovine haemoglobin solution,’ explains Zafirelis. Blood is taken from a dedicated herd and the red cells broken to extract the haemoglobin. This is purified, then ‘polymerised to a molecular size that is large enough to avoid escaping through the endothelium, but not too large to present a target for the immune system’.
When an organ is removed from a body it is immediately starved of oxygen. The standard procedure is to cool it in an organ preservation fluid until it’s transplanted. The cold reduces the cells’ metabolic rates, limiting damage due to lack of oxygen. But none of the approved organ preservation fluids contain a source of oxygen. ‘There some good data showing that perfusing organs with a couple of these oxygen carriers can preserved organs much longer than the current non-oxygenated solutions allow,’ says Jahr. This allows more time to find and reach a suitable transplantee. ‘In addition, there are articles showing that using these on transplanted organs that would previously have been deemed of unacceptable quality have shown very good survival, not only of the organ but of the person that it was transplanted into.’4,5
In October 2017, HbO2 Therapeutics announced the first successful transplant of a liver previously deemed unsuitable for transplantation that had been ‘refurbished’ by Hemopure. This transplant took place at the University Medical Centre Groningen in the Netherlands. ‘We are now planning a larger clinical trial using Hemopure in liver transplants starting in 2018,’ explains Zafirelis. This will take place at the Queen Elizabeth Hospital in Birmingham, UK.
His team are also working with the Spanish National Centre for Cardiovascular Research. Pre-clinical work there is looking at infusing Hemopure into a coronary artery following a major heart attack, giving the heart an instant oxygen boost to help reduce damage. Zafirelis also says that ‘we have a couple of other indications that we are pursuing’, but is unable to reveal what these are.
From lugworm to clinic
Another company pursuing approval for an HBOC to be used in organ perfusates is Hemarina, based in Brittany, France. In November 2017, Hemarina announced that 60 successful kidney transplants had taken place after their product Hemo2Life was used to preserve the organs prior to transplantation. This was the second clinical trial for Hemo2Life, after 10 kidneys had also been successfully stored in an earlier trial. Hemarina have now completed enough trials to file for approval to use Hemo2Life in Europe, explains its chairman and co-founder Franck Zal. ‘We’re hoping to be on the market in 2019.’

Like Hemopure, Hemo2Life is an animal haemoglobin. ‘We use haemoglobin from a marine invertebrate called a lugworm,’ Zal says. Lugworms live buried under the sand on beaches, obtaining their oxygen from water during high tides. ‘I was very interested to understand how this worm can live [without water] between the high tide and low tide, so I focused my attention on finding its oxygen carrier,’ Zal says. What he found was a form of haemoglobin that is more than 50 times larger than its human equivalent.
‘These worms have a very large polymerised haemoglobin as their native haemoglobin,’ explains Jahr. They don’t have red blood cells; instead, the free haemoglobin moves around their bodies in a fluid. ‘When it gets to their surface, it oxygenates from the outside, and travels to the inner parts of the worm where it offloads its oxygen.’ Hemarina has a worm farm in west France that produces over 1 million lugworms per year. And unlike with bovine haemoglobin, the worm haemoglobin doesn’t need to be polymerised or modified in any way.
Hemarina plans to seek approval to use Hemo2Life outside of Europe, and for preserving other organs beyond kidneys. ‘We have already demonstrated efficiency with other organs including the lung, heart, liver and pancreas,’ Zal explains. The company has two other products containing lugworm haemoglobin in development: an oxygenating wound dressing called Hemhealing and a red blood cell replacement called Hemoxycarrier. The latter is in pre-clinical development for treating strokes, heart failure and stickle cell disease. ‘We hope to be ready to start Phase I trials with Hemoxycarrier in two years,’ Zal says. Hemoxycarrier is currently stored frozen, but the team is working towards a freeze-dried version to broaden its potential use.
From Russia with blood
Polymerised animal haemoglobins are not the only potential approaches to replacing donor red cells. A second generation of the now-withdrawn perfluorocarbon-based oxygen carrier Fluosol has been approved as a blood substitute in Russia since 1997. Perftoran has since been approved in a few former Soviet republics, explains Deborah Thompson, president and chief scientific officer of FluorO2 Therapeutics. It was also sold in Mexico for a few years under the name Perftec. Perftoran has reportedly been administered to more than 35,000 people to date, with good results and few adverse effects.6
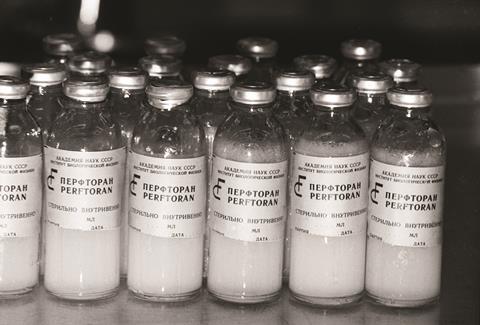
North Carolina-based FluorO2 Therapeutics owns the intellectual property rights to Perftoran in the US, where it is called Vidaphor. Perfluorodecalin, a perfluorocarbon able to carry and release oxygen, is the primary component of Vidaphor. It also contains a second, stabilising perfluorocarbon and an emulsifier. ‘The final product is a milky looking emulsion,’ says Thompson. ‘It is stable at five years frozen and when taken out of the freezer it’s stable in the fridge for two weeks.’
FluorO2 Therapeutics is currently developing a manufacturing process that meets the requirements for use in the US. ‘We then have a number of possible indications we’d like to explore in clinical trials in the US,’ explains Thompson.
Artificial red blood cells
Another potential approach is to encase an oxygen carrier in a polymeric shell. These products emulate the appearance and function of natural red blood cells more closely than the free haemoglobin or perfluorocarbon products. But none have yet reached clinical trials.
Kalocyte, based in Missouri, US, is one company pursing this approach. Its bagel-shaped Erythromer is in pre-clinical trials.7 ‘We encapsulate purified human haemoglobin in a synthetic polymer that forms an artificial membrane,’ explains Kalocyte’s president and co-founder Allan Doctor. ‘Inside the particle there are also two small molecules that modify the behaviour of the haemoglobin. One lowers the oxygen affinity, making it easier for the haemoglobin to release oxygen. The second is a reducing agent that keeps the iron in the haemoglobin from oxidising, extending the circulatory longevity of the particle.’
And the polymer doesn’t just act as a casing, it also controls the availability of the small molecule which assists oxygen release. ‘The small molecule is negatively charged, and the inner surface of the polymer has a positive charge,’ explains Doctor. ‘In the lung, where the pH is high and there’s very low proton concentration, the small molecule is sequestered on the inner surface of the cell, increasing its oxygen affinity.’ As the cell travels out into the body, the proton concentration around it goes up. The polymer then releases the small molecule, enabling it to interact with the haemoglobin – pushing the oxygen off. ‘We’ve got facilitated oxygen release,’ Doctor explains. ‘Erythromer is very effective at releasing the oxygen, especially when there’s an acid around, as in tissues with low oxygen levels.’
Erythromer is about 200nm in diameter, about one 50th of the size of a normal red blood cell, and can be freeze dried. ‘The first indication we’re seeking is in the pre-hospital care of massive trauma,’ says Doctor. He hopes to start clinical trials in three to five years.
The real deal
Blood pharming is another possible route to a donor blood alternative. Human red blood cells grown from donor blood stem cells in the laboratory would allow access to blood for patients with rare blood types, explains Ash Toye, a biochemist at the University of Bristol in the UK. Toye is taking part in a Phase I clinical trial of laboratory-grown blood scheduled to start in late 2018.

This trial – funded by National Institute of Heath Research and NHS Blood and Transplant – will test the longevity of laboratory-grown cells in the blood versus normal donated blood cells. ‘At the minimum we want equivalence,’ says Toye. ‘Our hope is, because they’ve been freshly made, they will have a long life span.’ The team is also looking at ways to scale up and automate the manufacturing process: currently they can produce 10ml at a time, enough for a baby, but far short of the volume normally used in an adult blood transfusion.8
Also, the blood cells are currently grown from adult donated blood but the team would also like to use umbilical cord blood. Cord banks hold a wider range of blood types than is typically donated, he explains. ‘There is a better representation of different ethnic groups within the population.’ But his long-term goal is to engineer grown blood cells to be universally compatible or to act as a therapeutic. ‘We want to make blood that has an altered function; such as having an enzyme which could be used therapeutically,’ he says.
With so-many different pathways to an alternative to donor red blood cells in clinical and pre-clinical trials, it’s easy to see why these researchers are hopeful that this field’s run of bad luck is coming to an end and an approval is just around the corner. ‘It’s almost unethical, when there are products that have been shown to be efficacious and to save lives, not to be using them,’ concludes Jahr.
Nina Notman is a science writer based near Salisbury, UK
References
1 J Posluszny and L Napolitano, Arch Trauma Res., 2016, 5, e30610 (DOI: 10.5812/atr.30610)
2 C Natanson et al, JAMA, 2008, 299, 2304 (DOI: 10.1001/jama.299.19.jrv80007)
3 C Mackenzie et al, Am. J. Ther., 2015, 22, e115 (DOI: 10.1097/mjt.0000000000000009)
4 A Matton et al, Liver Transpl., 2017, DOI: 10.1002/lt.25005
5 R Thuillier et al, Am. J. Transplant., 2011, 11, 1845 (DOI: 10.1111/j.1600-6143.2011.03614.x)
6 G Latson, Shock, 2017, DOI: 10.1097/shk.0000000000001063
7 D Pan et al, Blood, 2016, 128, 1027 http://www.bloodjournal.org/content/128/22/1027
8 S Kupzig et al, Haematologica, 2017, 102, 476 (DOI: 10.3324/haematol.2016.154443)












3 readers' comments