Investigating the heaviest elements known is rewriting our knowledge of chemistry and may even mean the end of the periodic table itself, writes Kit Chapman
In 2018, Peter Schwerdtfeger published a paper that turned chemistry on its head. According to calculations he and his colleagues performed, oganesson – element 118, the heaviest known – was not a noble gas as you would expect from its position in the periodic table, but a highly reactive solid. Even stranger, it didn’t seem to have electron shells.1
‘Well, that statement is oversimplified,’ says Schwerdtfeger, a theoretical chemist at Massey University in New Zealand. ‘You can still build up the electron densities from orbitals describing individual shells. What happens is that for oganesson the shell structure is barely visible, approaching an electron gas.’
While the calculations, done in partnership with researchers at Michigan State University in the US, have not been experimentally verified, they may not remain so for much longer., Experimentalists are already beginning to probe elements previously thought untouchable. And the results are departing from everything chemists expect.
A superheavy problem
Elements 104 and beyond are known as the superheavy elements. They do not exist on Earth, and must be created one atom at a time by smashing atoms of two lighter elements together in particle accelerators. It’s a process that costs hundreds of thousands of pounds in beam time. The typical production rate varies depending on the element; some are one atom a week, some are one atom a day. The elements’ properties are also a hurdle: all known isotopes of the superheavy elements are unstable and highly radioactive. In most cases, any experiment must be performed in seconds if it stands any hope of success.
Despite the difficulty, experiments to confirm the superheavy elements’ place on the seventh row of the periodic table have taken place almost since they were first discovered in the late 1960s. In 1970, a team at the University of California, Berkeley, US, were able to run atoms of rutherfordium-261 through an ion exchange column and demonstrate it was a homologue of titanium.2 The whole experiment – from creating a single atom to isolating it and running it through the apparatus – took less than a minute.
As the technology advanced, so did the complexity of what could be done. For example, the first chemical experiment on dubnium, element 105, saw atoms attached to potassium chloride aerosols, collected on a glass plate, fumed and washed with nitric acid and investigated with spectroscopy. Dubnium adsorbed on glass, following the trend of lighter group 5 elements.
In the 21st century, faster experiments and better particle accelerators capable of producing and detecting atoms more regularly have tested the chemistry of the superheavies on a regular basis. While only the most basic experiments can be attempted, even these can reveal a lot about an element. For example, in 2014, a team working at Riken in Japan – the lab credited with the discovery of nihonium, element 113 – bombarded a curium-248 target with neon-22, creating atoms of seaborgium, element 106, before separating them from the beam using magnets and treating them with carbon monoxide. The resulting molecules were then funnelled into a silica gas chromatography column lined with radiation detectors. With just 18 atoms, the team was able to confirm seaborgium had formed a Sg(CO)6 carbonyl, exactly like its lighter homologues molybdenum and tungsten. By heating the hexacarbonyl, the team then destroyed the bonds and calculated the bond strengths. Direct mass measurements of the heaviest elements have also succeeded, calculating an atom’s mass-to-charge ratio from where and when an atom impacts inside the detector.
The most challenging work comes at the far end of the seventh row of the periodic table, where the elements are diverging from their expected properties. As the number of protons and neutrons in a nucleus increases, so does the nucleus’ effect on nearby electrons due to quantum mechanics. This brings nearby electrons into a closer orbit, increasing their speeds – and mass (thanks to relativity). It’s the phenomenon that gives gold its unique colour, or makes mercury a liquid at room temperature. ‘It’s clear that for main group superheavies [elements 113–118], and for the late transition metals roentgenium and copernicium [111 and 112], relativistic effects are very important,’ Schwerdtfeger explains. ‘For roentgenium and copernicium, we have strong relativistic s-subshell stabilisation, and for the p-block elements we also have a strong spin–orbit coupling in the p-subshell. This leads to large changes in physical and chemical properties.’
The future looks bright for atom-at-a-time chemistry and spectroscopy
Robert Eichler, head of laboratory radiochemistry at the Paul Scherrer Institute in Switzerland, has used ultrafast techniques to investigate the chemical properties of copernicium and flerovium. He calls Schwerdtfeger’s calculations a ‘game changer’ in how experimentalists have approached the elements. ‘The lower the ionisation potential and the electron affinity, the more metallic an element is,’ he explains. ‘The cohesive energy is equivalent with the sublimation enthalpies that we chemists use to describe the volatility of an element or compound.’
Speed means Eichler’s experiments must be kept to simple gas–solid chromatography. The moment an atom is created, it is separated and fired down an array of detectors with a temperature gradient. By measuring where the atom adsorbs, its sublimation enthalpies can be calculated. These properties generally follow periodic trends, although with noticeable shifts. Eichler has found the behaviour of flerovium points to a higher level of inertness compared with its nearest homologue, lead; it still behaves like a metal, but only barely.3 The problem is that, given only around 100 atoms of flerovium have ever been created, experimental certainty is hard to achieve. Other teams are attempting to replicate their findings, or to find new ways to probe flerovium, such as trapping it inside a thiacrown ether.4
Such techniques could, in theory, be used for even heavier elements. ‘We now have at our hands a prediction for oganesson, suggesting its volatility is between mercury and copernicium,’ Eichler says, referring to a recent paper from Schwerdfeger that suggests oganesson is a semiconductor.5 ‘Therefore, we could apply similar experimental strategies to assess the character of oganesson.’ Schwerdtfeger agrees: ‘The future looks bright for atom-at-a-time chemistry and spectroscopy. One could trap an atom and perform spectroscopic experiments in the second or less time frame.’ The challenge, both concede, is that at the moment even his rapid experiments aren’t fast enough. And, even if he could speed up his tests, separating the newly formed atom from the debris found in a particle accelerator still takes a tenth of a second – 200 times longer than the half-life of oganesson’s sole known isotope.
The next steps
While oganesson is out of reach for now, no one is conceding defeat. One of the main obstacles to attacking this end of the periodic table is the limited number of atoms, so a major step is to scale up production. At the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, the Superheavy Element Factory aims to speed up the process of making atoms. Launched earlier this year, the new facility is capable of firing 60 trillion ions a second at a target – a beam 10 times more intense than its nearest rival.

The main motivation for the Superheavy Element Factory is to create the undiscovered elements 119 and 120 in collaboration with teams from around the world. But the machine is also capable of furthering our understanding of the existing elements, too. ‘The accelerator is built to create the isotopes we know and love,’ says Mark Stoyer, from Lawrence Livermore National Laboratory in the US, and part of the team that discovered elements 114–118 at JINR. ‘The idea is that if you can produce more of a given isotope, you can do other kinds of experiments than just watch it decay. Instead of having to wait a week, or a couple of weeks, you can now make tens of atoms, so you can do everything much faster.’
Tens of atoms doesn’t sound like much, especially when their half-lives mean they decay in less than a second. But Stoyer believes the Superheavy Element Factory will open new options for chemists. ‘Let’s take livermorium,’ he says, referring to element 116. ‘There are four isotopes, with half-lives in the tens of milliseconds. You’re very unlikely to get two atoms at a time, but what you can do is put them next to chlorine, oxygen or hydrogen and see how they interact. That gives you clues as to the chemistry.’
One of the main issues with making elements is that the technique, dubbed hot fusion, involves the loss of neutrons, throwing them away like ballast to try and reduce the energy and stabilise the newly formed nucleus. However, for elements such as flerovium, more neutron-rich isotopes are much longer-lived. For example, while flerovium-289, created when three neutrons are lost during hot fusion, has a half-life of around 1.9 seconds, flerovium-290, when only two neutrons are lost, is stable for around 19 seconds. The more atoms are created, the higher the chance of producing flerovium-290 – making a wealth of rapid-fire reactions possible.
Everything we know and observe must be explained by an underlying theory
‘If you have 1000 times more production, you can put that into sensitivity – so making something that is 1000 times harder to produce,’ says Rolf-Dietmar Herzberg, a nuclear physicist at the University of Liverpool, UK. ‘If you choose your energy differently, you could look for the 2n [two neutrons lost] channel’.
‘In a fantasy world, we might get the 1n [one neutron lost] channel,’ Stoyer adds, hinting at the possibility of the undiscovered flerovium-291 – an isotope that could measure its half-life in minutes. ‘Then you have the chance of doing other things. Studying how the oxide forms, determining the oxidation states that the element likes to have. How many electrons does it like to lose? [The Superheavy Element Factory] may provide us the chance to study and expand this region a little bit more.’
Even if the flerovium experiment is successful, it’s still some distance from the team’s true goal: the island of stability. Flerovium-298 is believed to be the heart of a region of nuclear stability, caused by having filled shells (known as magic numbers) of protons and neutrons. Here, flerovium could theoretically exist with a half-life of more than a million years; the problem is that no one can come up with a reaction in the lab that would be able to create such a neutron-rich isotope.
Even so, these experiments will also give theoreticians a chance to hone or confirm their models. ‘Everything we know and observe must be explained by an underlying theory,’ says Schwerdfeger. ‘The so-called Standard Model in physics predicts properties of particles to an unprecedented accuracy. We have all the theory at hand to predict any property of any element in the periodic table in any aggregate state. The problem is that, as [physicist] Paul Dirac once said, the equations are difficult to handle. But enormous progress has been made in quantum chemistry over the past 20 years. We can calculate them to high accuracy … but it is always good to see how theory agrees with experiment.’
A messy future
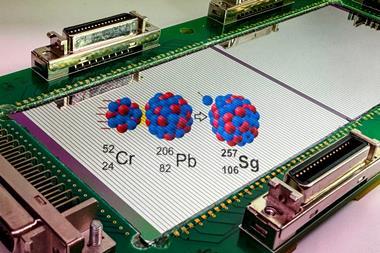
Currently, the team at JINR is attempting to create element 119 by firing a neutron-rich titanium-50 beam into berkelium; they are competing with a team at Riken, who are trying to forge the new element by firing vanadium into a curium target. Most researchers expect one or both to succeed within five years.
Yet while the superheavy element community is largely in agreement that elements 119 and 120 will belong in groups 1 and 2 of the periodic table, a question mark hangs over the elements beyond. Modern calculations put the point at which there can be no more elements because the nucleus cannot hold together at around element 172, suggesting there is still a third of the table left to discover. The problem is how they would map to the periodic table. Does element 121 belong in group 3? Does it form the beginning of a new ‘superactinide’ series? Or does it do something entirely different?
One idea, put forward by Pekka Pyykkö at the University of Helsinki, Finland, would see a completely new series – and with it a new electron shell. ‘In my proposal, elements 121–138 would formally form a “5g row”,’ Pyykkö says. ‘An electron shell of a new type, “g” or l = 4, would start to be occupied. As with the 4f shell, it would again be very compact, deep inside the atom, and for certain occupations, strongly magnetic.’
These would not belong with the lanthanides and actinides, but in another, separate box of its own. Pyykkö’s calculations would see his series rejoin the main periodic table with elements 139 and 140 in groups 13 and 14 respectively, before elements 141–155 fall into a new series under the actinides.6 Element 156 would thus be a homologue of titanium, creating a seemingly random jumble of elements as atomic number bows to the rules of shell structure and periodicity.
Pyykkö’s idea is far from the most drastic, too. For an element to be considered created, it currently requires a half-life longer than 10–14 seconds – roughly the time it takes for a cation to attract electrons. However, under the best theoretical models, it’s possible there will be a region of new elements with isotopes so unstable they cannot meet this category, instead existing as bare cations. Would we consider these nuclei part of chemistry, or solely something for physicists to worry about?
While it’s only a theory at present, if true it would once again turn everything we know on its head. The superheavy elements may break from Dimitri Mendeleev’s design, but it could be that the periodic table, chemistry’s most recognisable symbol and being celebrated this year, extends even beyond the central science itself.
Kit Chapman is a science writer based in Southampton, UK, and the author of Superheavy, published by Bloomsbury in June 2019 and reviewed for Chemistry World by Philip Ball
References
1 P Jerabek et al, Phys. Rev. Lett., 2018, 120, 053001 (DOI: 10.1103/PhysRevLett.120.053001)
2 R J Silva et al, Inorg. Nucl. Chem. Lett., 1970, 6, 871 (DOI: 10.1016/0020-1650(70)80067-8)
3 A Yakushev et al, Inorg. Chem., 2014, 53, 1624 (DOI: 10.1021/ic4026766)
4 J D Despotopulos et al, J. Radioanal. Nucl. Chem., 2016, 310, 1201 (DOI: 10.1007/s10967-016-4917-z)
5 J-M Mewes et al, Angew. Chem. Int. Ed., 2019 DOI: 10.1002/anie.201908327
6 P Pyykkö, Phys. Chem. Chem. Phys., 2011, 13, 161 (DOI: 10.1039/C0CP01575J)










No comments yet