Enzymes and metal–organic frameworks share some common features and are being investigated for industrial use. Clare Sansom reports
Frances Arnold from the California Institute of Technology, US, was awarded half the 2018 Nobel prize in chemistry for developing methods for the directed evolution of enzymes. Naturally occurring enzymes are some of the ‘greenest’ and most efficient catalysts known, and the evolutionary methods that she pioneered have greatly expanded the repertoire of reactions that can be catalysed by these exquisitely precise molecular machines. In introducing Arnold’s Nobel lecture, Sara Snogerup Linse of the Nobel committee for chemistry explained that Arnold has made a strong effort to make this planet a better place: ‘The enzymes she develops can speed up chemical reactions in a sustainable way, making less waste.’
In over three billion years since life first emerged on our planet, evolution has generated millions of enzymes to catalyse thousands of different reactions. In contrast, in the past 100 years or so, chemists have discovered and invented a much smaller number of catalysts for use in industry. Most synthetic catalysts are less selective than enzymes; they often operate best at high temperatures or in otherwise harsh conditions, and they may generate toxic by-products. It is not surprising that, by the mid-20th century, chemists were turning to the natural world for alternative catalysts, but as most reactions needed by the chemical industry are not found in nature, no enzymes have specifically evolved to carry them out. The technique that won Arnold her Nobel is, essentially, a way of choreographing evolution to produce enzymes capable of performing the tasks that we require.
I immediately realised the importance of what my group had done
Chemical synthesis in industry often takes place in highly polar organic solvents such as dimethylformamide (DMF). In the 1980s, German biophysical chemist Manfred Eigen suggested that evolution might be ‘directed’ to produce enzymes with specific functions, and in 1993 Arnold published a seminal paper describing the directed evolution of a bacterial protease, subtilisin E. ‘This enzyme was already widely used in detergents, but it was unstable in the highly “unnatural” chemical environment found in washing machines,’ she explains. ‘By repeatedly generating panels of mutant enzymes differing by one amino acid and selecting the variant with optimum properties, we produced a subtilisin that worked efficiently in the presence of DMF.’
This novel enzyme, which differed from wild-type subtilisin at only four amino acid positions, launched Arnold’s stellar research career and with it the enzyme engineering industry. ‘I immediately realised the importance of what my group had done, but it took my colleagues in academia a while to agree,’ she adds. ‘Industrial chemists, however, recognised straight away that this technique would allow them to solve practical problems by creating novel enzymes.’
The main hurdle with this fundamental technique is that protein sequences are almost infinitely variable, and a rationally constructed library of mutants will only sample a tiny amount of the possible ‘sequence space’. Arnold collaborated with Willem Stemmer, a Dutch biotechnologist who had invented a DNA recombination strategy known as DNA shuffling (or more picturesquely ‘sex in a test tube’), to dramatically increase the size of these libraries without increasing their cost. Stemmer was a serial entrepreneur involved in the founding of several successful companies including Maxygen to commercialise DNA shuffling in 1997. This company folded in 2013 but one of its spin-outs, Codexis, is still engineering custom enzymes using Arnold and Stemmer’s directed evolution methods, often to replace harsher, less specific synthetic catalysts.
Into industry
Enzyme engineering techniques are now widespread throughout the chemical and biotechnology industries, including in multinationals like Merck and Johnson Matthey. The Johnson Matthey group has been producing enzymes and other catalysts for the fine chemical and pharmaceutical industries since 2002. In 2010, they complemented their existing catalyst portfolio with the acquisition of a small German biotech company, X-Zyme, which specialised in producing enzymes to synthesise chiral alcohols and amines to a high level of purity. Since then, they have developed a large collection of industrially robust enzymes using a variety of techniques, to optimise enzymes to the exact specificity that their customers require.
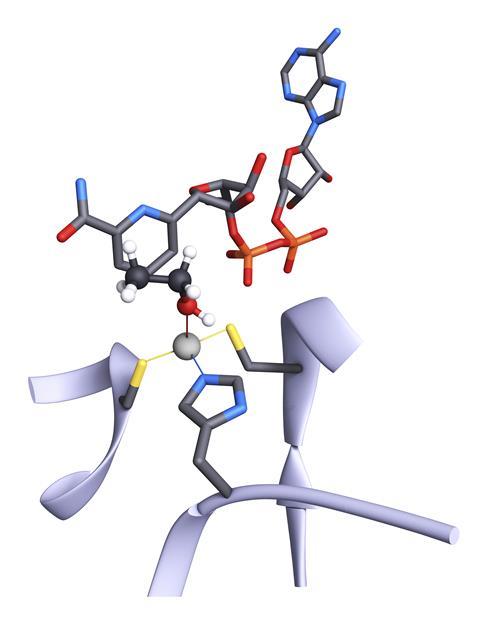
Hundreds of enzyme classes have actual or potential uses as industrial biocatalysts, but the field is dominated by just a few. Modern industrial biocatalysis really started with one of the most widely used classes: alcohol dehydrogenases. The function of these enzymes in mammals is to break down toxic alcohols, but the bacterial forms catalyse the ‘reverse’ reaction of alcohol synthesis, oxidising NADH to NAD+. It is these bacterial enzymes that are most industrially important, as efficient catalysts for the synthesis of enantiomerically pure stereoisomers of chiral alcohols. Johnson Matthey’s interest in X-Zyme began with a license that gave them worldwide exclusivity to commercialise the alcohol dehydrogenase in the bacterium Lactobacillus brevis.
The philosophy of Johnson Matthey’s enzyme offering is driven by their customers and the catalysts that they need. ‘Our first step always involves bioinformatics: a hunt for the most relevant enzyme sequence from among the 100 million-odd available in public and commercial databases,’ says Ahir Pushpanath, who leads the enzyme development arm of biocatalysis at Johnson Matthey in Cambridge, UK. ‘But if we do find a needle in this haystack, it is unlikely that its activity will be a perfect fit for the customer’s requirements; in that case, we try to tweak it with mutations discovered using sophisticated bioinformatics tools, including docking and molecular dynamics.’
Sometimes, however, these huge databases will contain no match that is ready for intensified process conditions. In this case, the Johnson Matthey team will use directed evolution techniques similar to Arnold’s to evolve the wild-type enzyme to new functionalities. ‘If enzyme engineering is to work efficiently, repurposing an enzyme that already has a tiny amount of the required functionality as well as its natural one will always be an advantage,’ says Pushpanath.
If you can’t find the enzyme with the exact specificity you need in the sequence databases or evolve it from one with a related function, you might, metaphorically speaking, find it under your feet. Novel enzymes are discovered almost every time a new bacterial genome is sequenced, and an enormous number of these genomes are still unknown. A typical soil sample contains many types of bacteria that have not been cultivated in isolation, but their DNA can still be sampled and sequenced using so-called ‘metagenomic’ techniques.
Soil samples from ‘chemically unusual’ environments are more likely to yield enzymes with novel functions and, perhaps surprisingly, it is not necessary to travel far to find these. ‘If you want to find enzymes that are thermostable, you need to look in harsh, hot environments.’ explains Serena Bisagni, a senior biochemist at Johnson Matthey in Cambridge. ‘These include active volcanoes and hot springs but can also be found much nearer to home; we found a very useful stable alcohol dehydrogenase in bacteria from a soil sample that had been constantly exposed to steam from a dishwasher.’
Interestingly, enzymes from bacteria that thrive in high temperatures are frequently also active at ambient ones. In fact, these enzymes will often work effectively enough for commercial use in conditions that are much milder than those needed by chemical catalysts. And they have one further advantage; they are soluble and active in water. ‘Most enzymes have evolved to function in the cytoplasm or the extracellular medium of living organisms, environments that can be approximated to an aqueous solution,’ says Pushpanath. ‘With biocatalysts, unlike with conventional ones, there is usually no need to run the reactions in “crazy” solvents that may be toxic, difficult to dispose of safely or just expensive.’
Enter the MOF
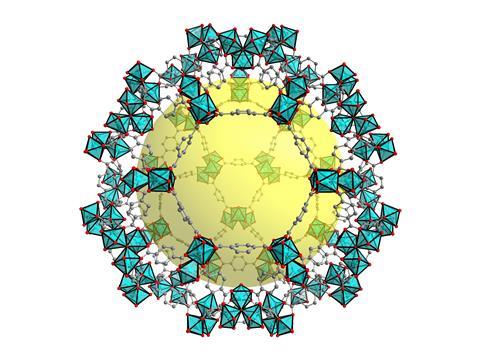
Many industrially useful enzyme families – including some classes of alcohol dehydrogenases – are metalloenzymes, in that their active sites contain one or more metal ions that play an important role in the catalysis. In alcohol dehydrogenases and almost all metalloproteases, that metal is zinc. These enzymes can be thought of as frameworks of amino acids to hold the metal ion and other reactive groups in their relative positions in the active site, so they can form a transition state with the reactants and speed up the reaction. At the atomic level, this is strongly reminiscent of the structure of synthetic metal–organic frameworks, or MOFs. These are coordination polymers that consist of organic linkers that coordinate to metal ions to form frameworks, most often in three dimensions. These complex and often porous compounds have come to have many industrial uses, and some combinations of metals and their surrounding linkers will form well-defined catalytic centres that can stabilise a transition state as effectively as a metalloenzyme active site can. And MOF catalysts are considered to have important industrial applications.
In contrast to enzymes, MOFs form active catalysts in the solid state, whereas enzymes work in solution. During a MOF-catalysed reaction, the reactants pass through the framework in the liquid or gas phase; this defines MOFs as heterogeneous catalysts, differing in phase from the reactants. ‘The advantage of a heterogeneous catalyst for industry is that the catalyst can be easily separated from the products after the reaction has taken place,’ explains Christoph Janiak from the Heinrich Heine University of Düsseldorf in Germany. ‘Enzymes, however, are homogeneous catalysts, in the same phase as their reactants: these often have higher activity and specificity, but the products are harder to purify.’
Another advantage of heterogeneous catalysts for industry is that they can be used in continuous processes. Simple solid-state catalysts are used in oil refineries to ‘crack’ long-chain hydrocarbons into the lower molecular weight fragments required by the oil and gas industries, without having to break the process to separate and recycle the catalyst. With a MOF catalyst, this type of process chemistry might be replicable in the synthesis of fine chemicals and pharmaceuticals. In contrast, processes involving homogeneous catalysts must be stopped from time to time and the products purified, both to prevent the loss of valuable catalyst and to remove potentially toxic impurities from the reaction product: the latter point is, of course, particularly important for pharmaceutical products.
The toxicity of a MOF catalyst is mainly determined by the nature of the metal that forms the framework or its active centre. Most metals that are catalytically active, whether in MOFs or in some other form, are to some extent toxic. Aluminium can be used to construct MOFs, and it has many advantages: MOFs incorporating aluminium are often stable and do not degrade, the metal ions are considered safe except at very high concentrations, and it is cheap and widely available – but aluminium has few catalytic applications. Platinum and other noble metals, which are much more widely used as catalysts, are cytotoxic and expensive. The exceptions, not surprisingly, are the metals that are regularly found in enzyme active sites. ‘Metalloenzymes have evolved to use the safest metals available – most frequently iron, manganese and zinc – but organisms have also evolved mechanisms to keep tight control of the amount of free iron and zinc in the body,’ says Janiak. ‘Even iron can be toxic in large enough quantities.’
A decade ago, expert commentators were predicting that MOFs would be joining enzymes in routine use as safe and effective industrial catalysts by the end of the 2010s. So far, however, most remain on the lab bench, apart from a very small number. ‘Our most serious concerns with MOFs as industrial catalysts are that they are often unstable in the presence of water, which is difficult to fully remove from industrial-scale synthetic chemistry, and that they are not thermally stable up to the effective reaction temperature,’ says Janiak. ‘There will be applications where this is not a problem, or even where an innate instability adds functionality, but these will be specialist ones.’
In the meantime, the protein engineering industry that has grown from Arnold’s inventions is producing specialist enzymes for an ever-widening range of commercial uses. ‘We are increasingly able to discover or engineer the perfect enzyme for a reaction, and set it up to work effectively under gentle, safe conditions,’ says Pushpanath. ‘And we are even beginning to use enzymes to replace heterogeneous catalysts in some continuous flow processes.’
Clare Sansom is a science writer based in London, UK
Article updated 28 March to correct the lactobacillus species












No comments yet