Nina Notman takes stock of the first products containing metal–organic frameworks to hit the shelves
Metal–organic frameworks, better known as MOFs, are coming of age. Their numbers have been mushrooming at an unprecedented rate since US chemist Omar Yaghi uncovered their potential nearly 20 years ago, and today the structures of over 6000 new MOFs are published each year. As their numbers have grown, so has the realisation that these highly porous materials have huge commercial potential, with touted applications ranging from methane fuel tanks in cars to antimicrobial dressings. Many academics working in this field have developed strong links with industry or have spun-out companies in a quest to help the materials meet their potential. And the second half of 2016 saw MOF applications dip their toes into the open market for the first time, with a handful of different products hitting the shelves.
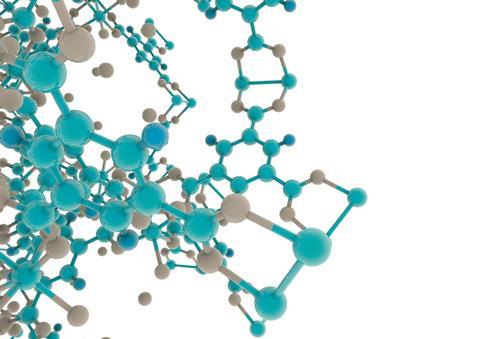
MOFs are metal ions or clusters held together by organic linkers to make highly ordered, crystalline 3D structures with ultra-high porosity. The phenomenally large internal surface areas inside these pores are one of the reasons for the buzz around these materials. ‘MOF porosity can reach up to 10,000 m2/g,’ explains Yaghi, a chemistry professor at the University of California, Berkeley in the US. To put that in context, other porous materials such as carbon, zeolites and mesoporous silica max out at around 1000 m2/g. Internal surface areas of MOFs are often given as comparisons to sports fields to help people visualise quite how large they are: 10,000 m2/g, for example, means that one gram of the MOF has an internal surface area approximately equal to 1.5 football (soccer) pitches or two American football fields.
Huge numbers of pores and the resulting large surface areas makes gas storage an obvious application. ‘Gases such as hydrogen, methane and carbon dioxide like to occupy large volumes but when in a MOF, by virtue of the interaction of these gases to the adsorptive sites within the pores, you can compact the gas molecules together much closer than they would be in the gas phase,’ Yaghi explains. This means, for example, that a gas tank in a natural-gas-powered bus containing a MOF can store more methane inside it than one the same size without the MOF. In turn this means the vehicle can travel further between each fuel stop.
Another reason for these materials’ appeal is how simple it is to design a MOF to behave exactly as you want it to. MOFs are programmable, explains Omar Farha, a chemistry professor at Northwestern University in Illinois, US. ‘There are only a handful of families of materials such as this where you can draw one on the board, go to the lab, make it and test it, and in most cases, your prediction is not far from reality.’ And the number of different components available to construct new MOFs from is almost unlimited. ‘We have a whole periodic table to choose from and we are building on the organic chemistry toolbox, so we have almost an unlimited number of organic linkers now too,’ Farha says.
First step
In 2012, Farha co-founded the spin-out company NuMat Technologies to commercialise his academic MOF research. And in autumn 2016, the company announced the launch of their first product line: ION-X. These gas cylinders contain proprietary MOFs holding very toxic gases such as arsine, phosphine and boron trifluoride. ‘These gases are used as dopants in the electronic industry,’ Farha says, and are typically stored in cylinders under high pressure. In the ION-X cylinders, the gas molecules are stored below atmospheric pressure meaning a vacuum needs to be applied to suck them out. ‘If you store these gases at high pressure and you have a leak, things will flow from the cylinder outward. However, by storing them sub-atmospherically, gas molecules will rush into the cylinder and not the other way around,’ he explains.

Farha was not able to say which electronic manufacturers are now using ION-X, nor give a ballpark figure of the kind of volumes his company are currently supplying. NuMat has however made public a collaboration with global gas supplier the Linde Group to develop MOF-based next generation separation and storage technologies – but as yet none of these have hit the market.
Another MOF product launched last year was TruPick, developed by spin-out company MOF Technologies in collaboration with Decco, a global company with expertise in fruit and vegetable storage. The pores in TruPick’s MOFs hold the gas 1-methylcyclopropene (1-MCP). ‘1-MCP retards the ripening of fruit,’ explains Stuart James, chair of inorganic chemistry at Queen’s University Belfast in the UK and co-founder of MOF Technologies. ‘Ethylene is a plant hormone, and when it is released by fruit it triggers ripening in other fruit. What 1-MCP does is bind to the ethylene binding site in the enzymes in the fruit and block the effect of ethylene.’
1-MCP gas has been used for over a decade to lengthen the shelf life of fruit and vegetables in warehouse storage containers, but this is the first time it has been supplied in a way that eliminates the need for gas cylinders. TruPick is a disposable sachet, which looks very similar to the silica gel desiccant packs put in shoe boxes, that when put in water releases the 1-MCP gas into the air. According to MOF Technologies, within 24 hours the released 1-MCP will effectively block the effects of ethylene on all the fruit in a storage room. TruPick is now approved for use, and being sold for use on hard fruits in the US and Turkey, and is going through the registration processes in other fruit-growing parts of the world including the EU.
Branching out

MOF Technologies has a number of other collaborative projects in the pipeline. Founded in 2012, the company was set up to commercialise the mechanochemical synthesis of MOFs invented in James’ academic lab. ‘One of the things that’s been holding back the implementation of MOFs has been developing cheap, scalable ways of making them,’ explains James. ‘What we found out was that, in some cases, you could make them very easily without using any solvent, which greatly reduces cost and makes the process more sustainable. You’d just take a metal salt and an organic ligand, and simply grind them together.’ The company currently makes 10 MOFs on a large scale using this method, explains chief executive Paschal McCloskey. ‘We’ve got a pipeline of another 20 or so MOFs.’
In 2015, McCloskey’s team started a three-year collaboration with computer giant IBM looking to use MOFs to collect the vast amount of heat generated in computer data centres and use it to heat and cool buildings. Here it is the thermal conductivity and thermal stability capabilities of MOFs that are of interest. ‘They can store large quantities of heat in a very, very small space,’ explains McCloskey. ‘Heat is a valuable commodity that can be recirculated for other purposes.’ Adsorption heat pump systems, powered by heat rather than electricity, already exist for this purpose but it is hoped MOFs-based systems will outperform the status quo.
Antimicrobial coatings for healthcare products are the third type of MOF products to have recently hit the shelves, developed by MOFgen, a spin-out from Russell Morris’s lab at the University of St Andrews in the UK. MOFgen is partnering with medical device companies to develop MOF-containing coatings for indwelling devices such as urinary catheters and textiles such as hospital gowns. ‘The MOFs act as reservoirs for antimicrobial agents,’ explains Yvonne Davies, MOFgen’s chief executive officer. ‘We can load them with biologically active gases, therapeutic agents and metal ions.’
When the MOFs first come into contact with water, or body fluids, the release of the antimicrobial agents is initiated. ‘For example, we can deliver a burst of nitric oxide gas that kills the area of infection to start with, and then a slow-release of an antimicrobial agent to help keep the infection away,’ she says. These smart coatings contrast the passive design of currently available antimicrobial coatings on these types of healthcare products. Davies is unable to confirm who MOGgen’s medical device partners are or what kind of volumes of MOFs the company is currently supplying.
Partnering with the big guys
In 2000, long before other academics started spinning MOF companies out from their labs, Yaghi began working alongside chemical giant BASF to explore the commercial potential of MOFs. To date nothing has hit the shelves yet, but a number of different products are in the pipeline.
We had a methane-fuelled MOF car fleet, but when the oil price dropped sharply, the economic incentive was no longer there
The currently mothballed project that has progressed the furthest forward to date is using MOFs in tanks for methane-powered vehicles. ‘We can store double or triple the amount of natural gas in a tank containing a MOF compared to a tank that does not, under the same temperature and pressure,’ Yaghi says. ‘A fuel tank [containing a MOF] has been tested with the methane uptake and release cycled to many, many cycles, to be equivalent to the lifetime of the automobile without seeing any degradation in the MOF behaviour or properties.’
The project was very successful technically, agrees Ulrich Mueller, who leads the MOF research group at BASF in Ludwigshafen, Germany. ‘We had a car fleet here at Ludwigshafen and we had a truck in the US running from east coast to west coast,’ he says. ‘But when the oil price dropped sharply, the economic incentive was no longer there.’ The cost for natural gas compared to liquid fuel is not currently significant enough to make methane-powered vehicles appealing to consumers. ‘This work is on hold and maybe if the situation changes we will pull it out from the drawer again.’
While the search for a viable commercial application may still be ongoing, BASF do already have a small business selling small volumes of MOFs to universities and institutes for research purposes. As you’d expect, BASF have various patents to protect these manufacturing processes and one process of particular interest is an aqueous synthesis. ‘We developed a so-called waterborne synthesis of some MOF materials, which are both sustainable and bring down with the costs of manufacture,’ Mueller says.
A separate problem
The applications discussed so far all use MOFs to first store and then release various atoms or molecules. But another interesting and potentially commercially viable use for these materials is in separating and capturing specific molecules from a gaseous mixture. Mosaic Materials, a spin-out from Jeffrey Long’s lab at the University of California, Berkeley, is looking to use MOFs to separate carbon dioxide from various mixtures, including capturing it from the flue gas emanating from power plants.
Carbon dioxide separations, using amine solutions, are already commonplace industrially. The largest and most established use of this so-called scrubbing technique is to remove carbon dioxide from, and therefore improve the quality of, natural gas. But it is also seeing use in carbon capture storage at power plants. ‘[Scrubbing] is a really huge industrial separation process that’s expensive and costs lots of energy,’ explains Long. ‘This technology could potentially be replaced by a much more efficient separation process using MOFs.’
For the last 15 years, the elephant in the room has been “Where are the applications?”
The main issue with the status quo is the amount of energy needed to get the carbon dioxide back out of the amine solution so that it may be reused. ‘Regenerating the amine solutions requires diverting high temperature steam that would normally be effective in making electricity in the power plant and using it to regenerate the capture solution,’ Long says. ‘These [MOF] materials can be regenerated at much lower temperatures, and so here you’re going to be utilising steam that’s not very useful in generating electricity and therefore dramatically lower the energy penalty associated with carbon capture in a power plant.’
Mosaic Materials was established two years ago to commercialise a cooperative binding method developed in Long’s academic lab. ‘We discovered a mechanism and a material for binding carbon dioxide, where when the first molecule of carbon dioxide absorbs it triggers the absorption of more carbon dioxide,’ he explains. ‘In our MOF, carbon dioxide is inserted between a metal site and a bound diamine molecule. That insertion triggers a proton transfer to a neighbouring metal diamine site which activates it. This results in a chain reaction to form 1D chains of ammonium carbonate units running along the channels within one of these porous materials.’ Warming up the material, or dropping the pressure, removes the gas and regenerates the original MOF structure. ‘These materials can be cycled many, many times,’ Long says.
Since the company was established, it has demonstrated the production of the MOF can be scaled up in an economical way and that it can be produced in a robust pelletised form. Mosaic Materials expect to be ready to start selling these and other MOF materials within the next year, says Long.
Long’s overarching concept, to functionalise the pores of MOFs to make them want to react with specific molecules only, is being explored by others for a broad range of different applications. According to Yaghi, one of the most interesting of these is using the pores to mimic the active sites of enzymes. ‘You can functionalise the pore to effect very specific bond-breaking and bond-forming reactions,’ he says. ‘This approach will be commercialised eventually, because doing catalysis in this way combines the robustness of heterogeneous catalysts with the selectivity of homogenous catalysts in a hollow material, which is sort of the holy grail of catalysis.’
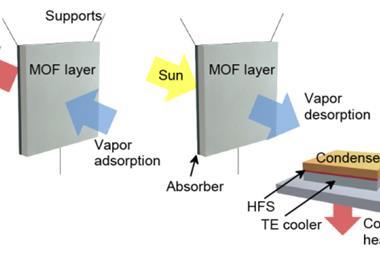
Another application that Yaghi is excited by the potential of is using a MOF to capture water from the atmosphere. He is just starting the process of commercialising a MOF developed in his group for this purpose. ‘This is a really powerful application, because we have demonstrated that you can take up water from the atmosphere and concentrate it and deliver it as drops of liquid water in an efficient way, because the water is not bound too tightly to the pores, like it would be in your dehumidifier,’ he says. ‘You don’t have to plug in this unit to make it operational; it can operate on just ambient sunlight.’
As the potential applications of MOFs continue to broaden, it is gratifying and reassuring for the researchers to see the materials start to hit the shelves. ‘To me for the last 15 years, the elephant in the room has been “Where are the applications?” There had been a lot of interest and some very, very good science done. But there hadn’t been this breakthrough in terms of actual uses. Now, in this last year, we have them,’ says James.
Nina Notman is a science writer based near Baltimore, US







No comments yet