Materials to safely encapsulate transplanted cells for could enable a revolution in the treatment of diabetes and a wide range of other diseases. James Mitchell Crow reports
Few medical discoveries have the lasting impact of the discovery made by a small multidisciplinary research team in Toronto, Canada, 100 years ago this month. In November 1921, the team isolated a substance from the pancreas that could bring blood sugar levels under control. By May 1922 Eli Lilly had begun mass production of the substance – named insulin.
Type 1 diabetes (T1D) onset usually occurs during childhood. Before insulin injections, few children who developed the disease lived for longer than a year or two after their diagnosis. By the start of the 20th century, researchers had established that T1D arose when a mystery substance stopped being produced by the islet cells of the pancreas. Animal pancreatic extracts had even shown potential for glucose control in diabetic patients, although they caused side effects too severe for regular use.
Canadian surgeon Frederick Banting suspected that problems with the pancreatic extract may be related to the fact the pancreas also produces the protein-digesting enzyme, trypsin. In the labs of University of Toronto physiologist John Macleod, Banting and a small team developed a method to kill off the enzyme-producing pancreatic cells, leaving the islet cells from which to extract insulin.
The discovery turned diabetes from a severe, fatal disease into a milder, manageable condition – a discovery worthy of the 1923 Nobel prize for physiology or medicine. As noted at the time, however, work remained to be done. ‘We must not imagine that insulin is able to cure diabetes,’ noted Nobel committee member John Sjöquist in the Nobel award ceremony speech.
Even with the latest insulin pump technologies, T1D patient glucose levels are imperfectly controlled. Over the long term, blood sugar fluctuations put people with diabetes at elevated risk of health conditions ranging from sight loss to chronic foot ulcers. Insulin injection technologies simply can’t match the responsiveness, efficiency, and accuracy with which pancreatic islet cells release insulin to keep blood sugar levels tightly regulated.
The primary challenge has always been that transplanted cells are subject to immunological rejection
So a growing number of scientists are turning to the cells themselves, developing materials-based strategies to enable the implant of functioning islet cells for the ultimate long-term control of the condition. If these strategies prove to be safe and effective, then people with a wide range of other health conditions, from osteoporosis to cancer, could also be set to benefit.
Suppression orders
Attempts to treat diabetes using implanted islet cells have a long history, notes Alessandro Grattoni, a biomedical engineer at the Houston Methodist Research Institute in the US. ‘Transplanting cells has been of interest for many years, in particular for diabetes,’ he says. Attempts to transplant islet cells are reported as far back as 1893, predating the discovery of insulin by almost 20 years.
‘The primary challenge has always been that transplanting cells is similar to transplanting organs: they are subject to immunological rejection,’ Grattoni says. The immune system quickly destroys the implanted cells.
Many immunosuppressive agents are actually toxic to the islet cells themselves
One option, now used widely, is to treat the islet cell transplant in the same way as a whole organ transplant, with recipients taking antirejection immunosuppressant drugs for the rest of their lives. In 2000, a team at the University of Alberta in Edmonton, Canada, pioneered an islet cell implant procedure strategy in which the cells were infused into the main portal vein of the liver. The Edmonton Protocol has since been carried out more than 1500 times worldwide, and some islet cell recipients have maintained insulin independence for a decade or more.
However, this approach has significant downsides. ‘Unfortunately, antirejection therapeutics have severe adverse effects,’ says Grattoni. The patient is immunocompromised, leaving them at higher risk of infectious diseases, as well as to developing certain tumours.
‘In the context of islet cell transplantation, immunosuppression is nasty,’ says Andrés García, a bioengineer at Georgia Institute of Technology, US. ‘As well as very serious consequences to the recipient, many immunosuppressive agents are actually toxic to the islet cells themselves,’ he adds. ‘There is evidence that some of the agents may result in insulin resistance, so it is not only bad for the patient but is affecting the graft as well.’
García and Grattoni are among the growing group of researchers exploring novel strategies to keep the implanted islet cells safe from immune system attack without resorting to antirejection drugs.
Barrier method
Rather than resorting to wholesale immune suppression, an alternative strategy is to keep the implanted cells and immune cells separated within the body. ‘The original attempts were to try to protect the cells within a physical barrier that would prevent immune cells from the host from coming and destroying the implanted graft,’ Grattoni says. The idea was to wrap the islet cells in semipermeable encapsulant materials that allow molecules, but not cells, in and out. The implanted cells could therefore perform glucose sensing and insulin release, and receive the nutrients and oxygen they need, while remaining safely out of the reach of immune cells.
One of the most thoroughly explored encapsulant materials is alginate, a polysaccharide derived from seaweed or algae that can be readily crosslinked to form a gel encapsulant for clusters of islet cells. Attempts to implant alginate-encapsulated islet cells date back to the 1960s. ‘There was some promise to this approach, it is explored to this very day,’ Grattoni says.
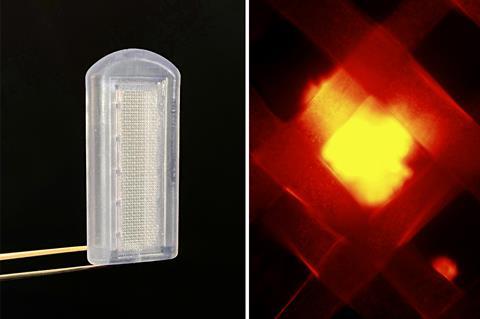
Alginate islet cell microcapsules, and related biomaterial encapsulants, work very well in diabetic mice, García notes. ‘Much work, for decades, has shown those approaches work well for small rodents, but they really face challenges as you move into larger animals,’ he says. But they must also overcome the body’s response to the implant.
‘One big challenge is that all materials are considered foreign in the body,’ says Minglin Ma, a biomaterials bioengineer at Cornell University in New York state, US. ‘Foreign material will cause reactions in the body when you implant them.’ The reaction starts with an inflammatory response, as immune cells are drawn to the foreign material and attempt to break it down. In implants that resist this degradation, fibroblast cells begin to envelop the material and form a physical barrier to isolate it from the rest of the body.
Some materials trigger stronger reactions than others. The response also varies by species, but is notably stronger in larger animals and humans that it is in mice. The reaction causes significant discomfort to patients, is associated with various health complications, and contributes to implant failure by at least partially choking it off from the rest of the body. ‘When the device itself is fibrosed, those devices normally don’t work very well,’ says Ma.
In highly purified form, alginate tends to trigger a relatively mild foreign body reaction, says Ma, who has used the material for islet cell implant devices. But the reaction is not eliminated completely, he adds.
Foreign body reactions can exacerbate a third major complicating factor of islet cell implantation, which is that islet cells require an excellent blood supply. ‘The native pancreas is very, very well vascularised,’ says Ma. In the short term, that blood supply is important for rapid glucose sensing kinetics, to release insulin as soon as the person eats.
However, poor blood supply also has longer term issues for the islet cell implant. ‘These cells are highly metabolically active and need to have good access to oxygen, nutrients and waste disposal,’ García says. Cells receiving inadequate supplies of nutrients and oxygen gradually die off, shortening the working lifetime of the implant. ‘Recently, the field has realised the cells need direct vascularisation to perform well and survive long term,’ Grattoni agrees.
Despite the long history of work on islet cell implantation, no solution has yet been implemented that overcomes the complex problem of simultaneously keeping implanted islets highly integrated into the blood supply, yet protected from attack by the immune system without resorting to systemic immunosuppression, while avoiding triggering a foreign body response.
But a new wave of cell implant concepts and approaches appears poised to overcome these problems.
Making connections
To ensure adequate blood supply, the ultimate approach could be to implant encapsulated cells directly within the vasculature, as is now done with the Edmonton Protocol to inject unencapsulated cells into the main portal vein.
The challenge with this approach is that it the encapsulated cell implant must be small, explains Nathaniel Hwang from Seoul National University in the Republic of Korea. ‘With alginate encapsulation the diameter is usually around 500µm,’ Hwang says. ‘You cannot inject this into the blood vessel, it is too big. You need a very thin layer of encapsulation and there hasn’t been any successful approach for that.’
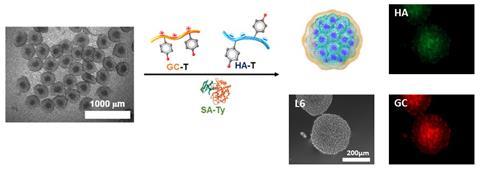
Hwang is working to overcome this problem using a multilayer hydrogel nanofilm to coat small clusters of islet cells. ‘The chemistry is very simple,’ he says. ‘The cell membrane itself is highly negatively charged, so you can apply a positive polymer and it will assemble a surface layer.’ Hwang then adds a negatively charged polymer, which self-assembles over the first layer, and repeats until the desired thickness is reached.
Using six layers of glycol chitosan and hyaluronic acid as the two polymers, the team created an encapsulation layer about 140nm thick. The key step to holding the whole construct together was enzyme-driven crosslinking. Each polymer incorporates phenol side chains, which can be converted into ortho-quinones using the tyrosinase oxidase enzyme.
The resulting cell coating proved to be robust, and selective in the molecules that could cross. Low molecular weight molecules such as oxygen, nutrients, glucose and insulin freely passed, but larger proteins were blocked. As a result, immune cells themselves, but also the inflammatory cytokines they release, were blocked from entry. In mice, the transplant kept blood glucose levels controlled for more than 30 days.
‘So far we have not seen our material cause any fibrotic reactions,’ Hwang says. The lack of fibroblast cells in the liver, as well as the use of hyaluronic acid as the outer layer, should help to minimise the foreign body reaction, he adds.
The encapsulant could be particularly suited to the transplant of islet cells sourced from animals such as pigs, an avenue being explored due to the acute shortage of islet cells from human donors. ‘If you can have complete shielding from the immune system, patients would not need to take immunosuppressants because the immune system will not recognise what is inside,’ Hwang says. The team is now working with a company working on animal-sourced cell transplants. ‘In order to be successful, I think this company may need our technology.’
Supporting local
Rather than implanting cells within the body’s vasculature, Grattoni and his team aim to bring vasculature to the implant. The team’s device is designed to be inserted under the skin, such as the inner arm, within which the islet cells would reside. An additional advantage to this approach is that the implanted cells remain fully contained and, if necessary, can be removed from the body by extracting the device.
The slow release of growth factors such as vascular endothelial growth factor can promote the growth of new blood vasculature into an implanted cell encapsulation device. But establishing new vasculature takes some time, which in the short term can place the implanted cells under stress due to the shortage of oxygen. ‘Your cells could be hypoxic for a duration of time, and that is really deleterious to long term performance,’ García says.
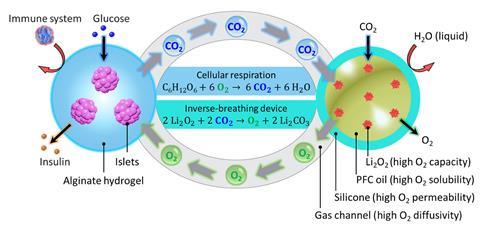
Ma recently demonstrated a device that incorporates an oxygen generation material to keep the cells happy when oxygen supplies are short. ‘We used lithium peroxide, the same material used in submarines when they don’t have enough oxygen,’ he says. Carbon dioxide released by the implanted cells during respiration reacts with the lithium peroxide, releasing a regulated supply of oxygen. When lithium peroxide particles were sealed into a silicone tube, and the tube was coated with alginate-encapsulated islet cells, the device restored glucose control in diabetic mice for at least three months. In a scaled-up version, the device also protected rat islet cells for two months when they were implanted into pigs.
Grattoni’s strategy is to implant the device first, and then add the islet cells later once the implant becomes vascularised. His 3D printed polymer implant incorporates a cell loading port to add the cells by subcutaneous injection, four weeks after implantation.
Although the device promotes excellent contact with the blood supply, the implanted cells are no longer inaccessible to immune cells. ‘We have conceived a device that contains two reservoirs, a cell reservoir and a drug reservoir,’ Grattoni says. ‘We load the drug reservoir with immune suppressants that are little by little eluted into the cell reservoir through a nanoporous membrane. This technology allows local immune suppression in the cell reservoir.’ Slow release of the drug across a silicon membrane punctuated with nanochannels is designed to achieve a sufficient local concentration of the drug to protect the implanted cells, while minimising exposure of the rest of the body to immunosuppressants. The drug reservoir can be refilled by needle as required. So far, the foreign body reaction has not caused significant problems in animal studies, Grattoni says. ‘Ideally, the implanted cells would last at the very least for 10 years,’ he adds. ‘We believe it is achievable if we can provide the right environment for the implanted cells.’
Sweet harmony
Instead of fending off immune system attacks, García is targeting immune system acceptance of implanted cells. By training key immune cells that the implanted cells should be protected, the implant will be left alone.
‘If we are able to induce immune acceptance of the graft, then we don’t need to encapsulate the cells,’ García says. ‘If you do not have to encapsulate the cells within a polymer, then you have the possibility that blood vessels can grow into the material to address all of these challenges of blood supply.’ The same approach could be used for many other biomedical cell implants beyond diabetes, he notes (see box Beyond diabetes below).
Beyond diabetes
Materials and devices designed for the transplant of islet cells to treat type 1 diabetes, enabling long term function of the cell while preventing immune system attack, should be equally suitable for the transplantation of a whole range of other cell types.
We are trying to encapsulate ovarian cells as a treatment for osteoporosis
One clear opportunity is to implant hormone-releasing cells – for example, thyroid-releasing follicular cells to treat hypothyroidism, testosterone-releasing lytic cells for male hypogonadism, or oestrogen-producing ovarian cells. ‘We are trying to encapsulate ovarian cells as a treatment for osteoporosis,’ Hwang says.
Other applications are more diverse. Of the many areas García is exploring, one is to develop materials that induce immune tolerance to whole organs. ‘We are beginning to do some work with solid organ transplant, things like kidney and hearts,’ he says.
Other potential areas where cell therapies have been investigated – and where cell-encapsulating materials or devices could play a key role – include chronic eye diseases, cardiovascular disease, neurodegenerative diseases and wound healing.
‘We think of our device as a local immunomodulation platform to activate or inactivate the immune system in different ways, depending on different diseases,’ Grattoni says. One area of research is cancer. Rather than keep immune cells away, the idea is to attract certain cells into the device. ‘We attract dendritic cells, and prime them with tumour antigen in order to trigger an immunological response to a cancer that we have extracted antigens from.’
García and his team have engineered polyethylene glycol based materials that they can decorate with proteins that promote immune tolerance. One of the proteins García is looking to exploit, PD-L1, has recently come to attention because it is expressed by many tumour cells to suppress immune system attack.
Rather than envelop the transplanted islet cells, the protein-presenting hydrogel is implanted alongside it. ‘What has been really fascinating is we have demonstrated you only need that immunoregulatory protein for a short window,’ García says. The team has shown the protein is cleared away within two weeks of implant. ‘The immune system gets trained to accept the graft, and from that point on you don’t need the protein anymore. A very potent subtype of the immune system called regulatory T-cells maintain the acceptance of the cells.’
Putting the concept into practice has worked better than he expected, García admits. ‘We have done this in mice out to 200 days, which in a lifespan of a mouse is considered long-term survival. We just completed a study in non-human primates that went out to six months with good outcomes,’ he adds.
Using a similar approach of displaying signalling proteins on a collocated hydrogel matrix, García has also shown it is possible to drive vascularisation around the cell graft. ‘We have found that when we present the protein in the context of our biomaterial, a sustained presentation, we end up having a stable vascular bed that persists for months.’
The team has combined both types of protein onto a single hydrogel, and is currently studying how the contributions of the vascularisation versus the immune acceptance lead to long term graft survival. The team – like Ma, and many in the field – is also actively looking at implants based on islet cells derived from stem cells. Producing islet cells from a stem cell bank would eliminate the bottleneck of needing to source cells from the limited supply of human donors. ‘We believe the future of islet replacement has to go through the stem cells, just because of the capability to manufacture the cell product,’ García says.
‘The idea that you can transplant islets with some immunomodulatory materials, and modulate the immune response locally and the islets are protected – I thought that was amazing,’ Ma says. ‘Islet cell transplant remains a difficult problem – but there is new thinking now.’
James Mitchell Crow is a science writer based in Melbourne, Australia











No comments yet