Is the cure worse than the disease? Katrina Krämer looks at the new alternatives to traditional dispersants and surfactants
20 April 2010 spelled disaster for the Deepwater Horizon oil rig. While drilling into a promising petroleum well off the coast of the US state of Louisiana, a sudden high-pressure burst of methane shot up the pipes and exploded. Flames engulfed the platform, killing 11 crew members. Two days later, the rig sank, leaving the well gushing crude oil 1600 metres below the surface. Over the next five months, 210 million US gallons of heavy crude spilled into the Gulf of Mexico – the largest marine oil spill in history.
Desperate to contain the spreading slick, Deepwater Horizon operator BP distributed more than 4000km of floating booms, placing some around entire islands. Hundreds of skimmer vessels were mobilised to scoop up the oil. Whole islands were constructed to protect Louisiana’s coastline. In a move that has remained controversial to this day, BP also purchased one third of the world’s supply of the dispersant Corexit. 771,000 gallons were applied directly at the broken wellhead.

Corexit is mixture of glycol ether surfactants, sorbitan emulsifiers, propylene glycol and light petroleum distillates. It breaks up oil into tiny droplets, intending to help natural oil-eating bacteria break down the hydrocarbons. But scientists soon started to uncover that dispersants might do more harm than good. ‘[Dispersants] only hide the problem,’ says Steven Pedigo, chief executive of bioremediation product producer Oil Spill Eater International. An outspoken opponent of dispersants, Pedigo accused the Environmental Protection Agency of violating US water pollution laws by approving dispersants to tackle the BP spill. ‘[Corexit] has the horrible reputation that it deserves.’
Dispersed oil, a 2014 study found, causes cardiac problems in tuna.1 To certain plankton species, a Corexit–oil mix is 52 times more toxic than either substance on its own, another study concluded.2 Dispersants could even cause harmful algal blooms – toxic red tides – by killing off the organisms that feed on the algae.3 Other experiments, however, showed no adverse effects on microorganisms. A 2017 investigation even concluded that dispersants cut harmful emissions from Deepwater’s slicks. ‘It’s a very difficult field scientifically,’ says science historian Ian Stewart of the University of King’s College in Nova Scotia, Canada. ‘Part of the reason why there is so much controversy is that it’s very difficult to model complex, large-scale ecosystems in a laboratory.’
Spiky solutions
Despite the controversy surrounding dispersants, scientists evaluating Corexit use during the Deepwater Horizon accident agreed that it should continue. It was the only way to minimise the already disastrous environmental consequences.

‘The industry is used to dispersants, and we could provide one that is less toxic,’ says chemical engineer Norma Alcantar from the University of South Florida in the US. Her team has developed a dispersant from cactus extracts that can compete with Corexit’s effectiveness but is essentially non-toxic. Alcantar’s research started with a story from her grandmother. She told Alcantar about a traditional Mexican method to remove sediments from water using the gluey substance found inside cacti. It turned out that the prickly pear cactus mucilage could also remove bacteria, heavy metals and oil from water.4
The mucilage – which can be dried and stored as a powder – contains a combination of over 40 amphiphilic sugars. ‘We have identified some of them: arabinose, galactose, xylose, glucose,’ says Alcantar. But simply combining these four sugars doesn’t yet make a dispersant. ‘Somehow the other sugars that occur only in tiny concentrations play a role,’ Alcantar explains. Toxicity tests with plankton confirmed that the cactus dispersant, unlike Corexit, doesn’t affect them.
Alcantar thinks that growing the cactus commercially is feasible. The plant grows fast and needs little water. ‘We think it’s very sustainable because it grows in desert-like environments where other crops don’t grow,’ she says. However, compounds made from natural resources can be a difficult sell if there is a cheaper, factory-made product on the market. ‘It’s a matter of economics. You have to have something that can be either produced super-fast or has a relatively long shelf life. The former is not easy to do if with natural extracts,’ says Nancy Kinner, director of the Coastal Response Research Center and an environmental engineer at the University of New Hampshire, US.
After three and a half weeks you couldn’t tell that there had ever been a spill
But even the most be benign dispersant doesn’t change the oil’s chemical composition – a feat Pedigo says his enzyme formula can achieve. The oil-eating mix contains biosurfactants – amphiphilic compounds like rhamnolipid – to break up slicks, as well as 156 different bacterial enzymes that pre-digest the oil. Added nutrients then speed up growth of indigenous microorganisms that convert hydrocarbons into carbon dioxide and water. A 550,000-litre spill in Nigeria that had coated 18km of shoreline proved an ideal testing ground for Pedigo’s enzyme formula. ‘After three and a half weeks you couldn’t tell that there had ever been a spill,’ he says.
Yet methods that rely on biological remediation often struggle with some of the most toxic oil components: polycyclic aromatic hydrocarbons (PAHs). Many PAHs are toxic, carcinogenic, teratogenic, or all of the above. Bacteria quickly break down small, water-soluble compounds like napththalene, but larger PAHs like benzo[a]pyrene can take decades to disappear. This is why researchers hope to find a way to remove oil from the environment entirely.
Mopping up the mess
‘The truth is, the vast majority of oil that gets spilled never gets cleaned up,’ says Seth Darling from Argonne National Laboratory in the US. In August 2010, four months after the catastrophic blowout in the Gulf of Mexico, only around 25% of the oil had been recovered. The other 75% remained in the ocean, as dispersed droplets, floating tarballs or buried in sediment.

By November 2010, almost 7000 large marine animals including birds, turtles and dolphins had turned up dead. Scientists predicted the hidden impact on smaller organisms like fish, plankton and deep-sea corals to be even greater. Persistent PAHs continued to wreak havoc on the ecosystem: the toxins are the likely cause for cancerous growths and congenital defects like missing eyes still seen in fish and shrimp years after the spill.5
A material developed by Darling, his colleague Jeffrey Elam and their team might one day be able to mop up slicks like a kitchen sponge;6 though unlike a regular sponge, it doesn’t take up any water. ‘The idea of the Oleosponge is that it can clean up surface slicks without any waste in principle, because you can collect and recover the oil and then reuse the sponge,’ Darling explains. ‘And it provides the first solution we know of to actually clean up oil out of the water column.’
Their discovery came about by chance. The team was using chemical vapour deposition to coat thin polymer layers with aluminium oxide, which makes the films more robust during lithography – etching microscale patterns into a surface to make microchips. Trying out this chemical vapour infiltration on a chunk of polyurethane foam – rather than a thin film – gave the team the idea of creating an oil-absorbing sponge.
Treating the foam with trimethylaluminum and water vapour first covers all of its inner and outer surfaces with a 10nm aluminium oxide layer. Onto this layer, the researchers then anchor the oleophilic (3-aminopropyl)triethoxysilane. ‘The trick is to make [the oil] bond strongly enough so you can actually pull it out of the water, but not so strong that it stays [in the sponge] permanently,’ says Elam. Van der Waals forces between the silane and hydrocarbon molecules hold the first priming oil layer in place. The oil’s hydrophobicity then does the rest, filling the sponge’s entire internal volume.
We were thrilled when the sponge performed beautifully
After many lab tests, Darling and Elam decided that it was time to put their sponge to the test in a real-world scenario, at the National Oil Spill Response Research Facility south of New York. But moving from bench scale to a two-million gallon outdoor saltwater tank was not straightforward, the researchers admit. The sponge had always been made as 1-inch cubes, but for their large-scale tests they needed 10,000 of them. ‘Because of the way sequential infiltration synthesis occurs, you can’t just scale up without changing anything,’ says Darling. ‘We had to learn through trial and error what conditions would work on larger batches.’
Darling remembers that ‘there were some trepidations going into these tests that it would not work at all’. Small-scale lab test often carry the risk of distorting the results, explains Darryl McMahon who leads the Canada-based Remote Energy Security Technologies Collaborative and advocates for new spill clean-up technologies. Surface tension, he explains, means oil behaves differently in small containers. ‘We were thrilled when [the sponge] performed beautifully,’ says Darling. Not only was it able to pull out different types of crude oil – one from the Alaskan north slope, one from the Gulf of Mexico – it even worked on diesel fuel, refined petroleum containing molecules with eight to 21 carbon atoms.
‘We’ve had a great deal of interest both from companies that want to use and evaluate it but also from companies that want to manufacture it,’ Elam reveals. ‘I think [the Oleosponge] has a lot of promise, but is currently many years from commercial availability,’ McMahon says.
Fake fur

‘They’re really fascinating to look at,’ says Hendrik Hölscher from the Karlsruhe Institute of Technology in Germany about a plant that inspired a different oil-absorbing material. ‘Under water they develop this silvery sheen on top of their leaves and if you take them out of the water they are immediately dry again.’ Hölscher and his team found that the superhydrophobic leaves of the floating fern Salvinia could be made into a biological oil sponge.7
While there are other superhydrophobic plants like lotus, they don’t usually absorb oil. But Salvinia’s leaves are covered with microscopic hairs. ‘It’s the space between the hairs that is available for oil absorption,’ explains Claudia Zeiger, a former PhD researcher in Hölscher’s team. The dried leaves could make for a cheap oil sorbent. ‘Salvinia is a pest plant in many regions, so this would be a win–win situation,’ she says. In northwestern Louisiana, the plants have become a nuisance, clogging up recreational lakes. ‘The only problem is that the backside of the leaf is not superhydrophobic when it’s dry, so it will absorb water as well,’ she says. The water cabbage Pistia could be a better option: its leaves are oleophilic on both sides.
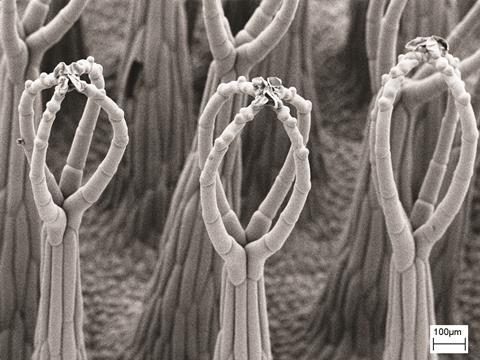
The team made an artificial version of this nanofur by pressing a heated rough steel plate into polycarbonate film. The melting polymer surface forms hair-like nanostructures when the steel plate is retracted. Although the artificial nanofur isn’t quite as effective at mopping up oil as the plant, scaling up its production should be straightforward, Hölscher thinks. However, ‘we haven’t managed to get an industrial partner yet to really get the project started,’ he says.
Progress through technology
The problem with all new technologies is that an oil spill is a low probability event, says Kinner. ‘It takes money to [create new technologies] and there isn’t really a huge demand for them. It’s a matter of whether these devices are a practical improvement. Is it something that can treat a very high flow rate? Is it something that can readily be deployed? Is it something that doesn’t foul easily?’ she says. ‘Dealing with accidents is not a revenue generator in the oil industry,’ agrees Stewart.

‘One of the biggest challenges is that there are so many different types of oil – from very light condensate to crude so heavy it sinks in water,’ says McMahon. ‘First responders often have no idea what they will find.’ This proved true during the Sanchi tanker accident in January 2018 – a frustrating experience for responders. The tanker was carrying 40 million gallons of condensate – a mix of short hydrocarbons such as pentane and hexane – when it collided with another ship. The Sanchi caught on fire, killing all 32 crew members and releasing its cargo into the East China Sea.
Dealing with accidents is not a revenue generator in the oil industry
This was the first time such an immense amount of condensate had been released. Condensate is more volatile than heavy crude, and some of its components are water-soluble. Cleaning technologies developed for heavier oils were useless. ‘Those spills are often problematic when it comes to exposure of workers or air-breathing marine mammals,’ says Kinner. Even monitoring the condensate’s spread proved challenging as it doesn’t form shimmering films.
‘Of course, in an ideal world, we wouldn’t have any spills,’ says Alcantar. But with most technology still dependent on petroleum, spills will – at least for now – remain a regular occurrence. In April 2018, the US government laid out plans for oil exploration in Alaska’s wildlife refuge – a move that worried residents and conservation groups. While controversial, situations like this could, says Stewart, ‘create a perfect storm of political, social, environmental and economic motives’ for researchers and companies to put their ideas forward for cleaning up after accidents.
However, it is uncertain if any ideas will turn into fully fledged technologies in time for the next spill. For now, mechanical removal and dispersants will remain the go to methods when it comes to dealing with slicks. ‘I think the oil industry will try to avoid trying any new technologies at any cost,’ says Restco’s McMahon. ‘I’m really hoping that we can change that.’
Article amended on 05 June 2018 to correct Ian Stewart’s affiliation
References
1 F Brette, Science, 2014, 343, 772 (DOI: 10.1126/science.1242747)
2 R Rico-Martínez, T W Snell and T L Shearer, Environ. Pollut., 2013, 173, 5 (DOI: 10.1016/j.envpol.2012.09.024)
3 R Almeda, S Cosgrove, E J Buskey, Environ. Sci. Technol., DOI: 10.1021/acs.est8b00335
4 D I Fox, D M Stebbins and N A Alcantar, Environ. Sci. Technol., 2016, 50, 2507 (DOI: 10.1021/acs.est.5b04145)
5 https://www.aljazeera.com/indepth/features/2012/04/201241682318260912.html
6 E Barry et al, Environ. Sci.: Water Res. Technol., 2018, 4, 40 (DOI: 10.1039/c7ew00265c)
7 C Zeiger et al, Bioinspiration Biomimetics, 2016, DOI: 10.1088/1748-3190/11/5/056003













No comments yet