Chemists are finding fascinating phase-change phenomena that make crystals jump and pop, learns Rachel Brazil
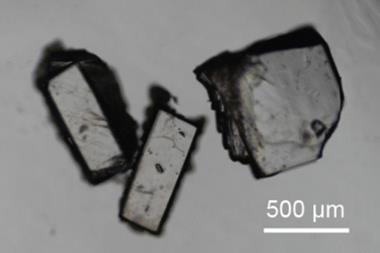
‘It started as a curiosity,’ recalls Pance Naumov, now associate professor at NYU Abu Dhabi in the United Arab Emirates. ’One of my postdocs came to me and said: “I can’t get hold of my crystals, they are escaping!”’ In 2009, Naumov’s solid-state laboratory at Osaka University in Japan was looking at crystals of oxitropium bromide (C19H26BrNO4), a drug used to treat asthma. ‘[The postdoc] discovered that when you put some crystals on the microscope, they start to jump … I was very surprised,’ says Naumov, but after a little digging he realised he was rediscovering a forgotten phenomenon – the thermosalient effect – where crystals respond to heat by self-actuation, or more simply put: jumping.
Naumov shared his finding with visiting chemical crystallographer, Joel Bernstein, now emeritus professor of Ben-Gurion University, Israel, who was familiar with the effect. Bernstein first saw the phenomenon at the University of Minnesota lab of chemist Margaret Etter. In 1983, she observed yellow (phenylazophenyl)palladium hexafluoroacetylacetonate (PHA) crystals fly off the microscope stage and turn red – the first publication on jumping crystals in the modern era.1 ‘She said: “Joel you’ve got to see this”. She marked a picture of a crystal projected on a big TV screen with a magic marker to show the size and then heated it. I saw that it expanded and jumped,’ remembers Bernstein.
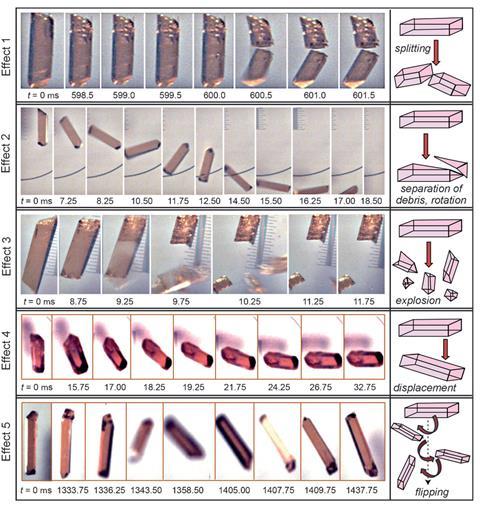
‘These phenomena had been reported before but were not investigated, because there were no means of looking at the structural mechanism,’ says Naumov. In addition, classic powder diffraction and thermal analysis techniques start by grinding up crystals, potentially destroying the evidence. A move away from optical microscopes and observation also explains modern chemistry’s lack of interest in this phenomenon. But the pendulum is swinging back now, says Bernstein. ‘People have started observing, recording, describing and researching dynamic phenomena.’
Naumov has been at the forefront of this movement. As part of this work, he has been searching published papers for examples of thermosalient crystals. ‘We had to go to the literature and look for hints that people had seen some kind of effect,’ he explains. The older literature, published at a time when data such a melting point were measured by observation, has proved particularly useful. Organic, organometallic and inorganic crystals exhibiting the effect were found, including oxitropium bromide, 1,2,4,5-tetrabromobenzene (TBB) and the cobalt complex [Co(NH3)5(NO2)]Cl(NO3), which suddenly jumped on excitation by UV light, labelled the photosalient effect.2
Questions answered
To understand what was happening Naumov took a closer look at the crystals studied by Etter under an optical microscope, coupled to a high-speed camera. He found the crystals were undergoing a rapid, self-perpetuated solid-state phase transformation. The phenomena is triggered by a temperature change that causes a molecular change leading to internal strain building up in the crystal lattice. Eventually the crystal undergoes a transition to an energetically more favourable phase. When one molecule switches from one crystal phase to another, it triggers the same change in a neighbouring molecule – at the rate of over one billion molecules per second.3 ‘The mechanical energy is released very fast, and what we are seeing is a consequence, the mechanical motion is just a result of this extremely rapid energy transduction,’ explains Naumov.
Far away from these molecular materials, there are structural parallels in a well-established phase transition seen in steels and a few other alloys. These so-called martensitic transitions are named after the 19th century German metallurgist Adolph Martens. When steel is cooled extremely fast, a very hard – but brittle – martensite phase is formed. The high-temperature face-centered cubic austenite iron lattice undergoes a transition to a highly-strained body-centred structure known as martensite. The structural change is subtle, a slight expansion in one direction and a contraction in the other two. But because the change is so quick, the carbon in the alloy does not have time to diffuse out and so the new non-equilibrium martensite phase forms with carbon atoms sitting between its iron lattice sites. It is this carbon that is responsible for steel’s hardness.
No jumping is observed in steels, but nevertheless Naumov believes that his jumping molecular crystals are undergoing a similar change from one solid state to another. He says it was surprising to find that such a subtle structural change was responsible. ‘For such a strong mechanical response you might expect that something really tremendous could be seen in the crystal,’ he explains.
Strong evidence to support a martensitic-transition explanation includes the sound emitted during the transition – like the popping noise of popcorn. In metal alloys this is known as an acoustic avalanche. In July 2017, Naumov published evidence of acoustic waves generated from a transforming crystal of the natural amino acid derivative, L-pyroglutamic acid (L-PGA), picked up by a piezoelectric sensor.4
These jumping crystals are more than just an interesting novelty though, they are potentially useful too, due to their unusual ability to convert external stimuli – such as heat or light – into kinetic energy. ‘This aspect is very fundamental because we are looking at alternative ways of energy transduction,’ says Naumov. To take advantage of these energy conversions, chemists will need to understand more about how these properties arise. Ultimately we want to be able to predict the relationship between structure and property – can we find the types of materials that will show this behaviour and then tailor their structures to optimise the desired effect? ‘I don’t think there is anybody in this field who can look at a molecule and say it will do this,’ says Bernstein, ‘I think we are a long way from that.’
Naumov has, however, succeeded in categorising thermo and photosalient crystals into three groups based on their structure and hydrogen bonding. Two of the groups don’t have any hydrogen bonding, and therefore often disintegrate or explode on phase transitions. The third group has hydrogen bonding in one or two dimensions. ‘These are the most interesting,’ explains Naumov, ‘they remain intact after multiple cycles.’
Materials with memories
Martensitic transformations provide another unusual property that has been found in metal alloys, shape memory effects. This is the ability of an alloy to be permanently deformed and then, on heating, recover its original shape. The most well-known shape-memory alloy is nickel-titanium (Nitinol). Its shape memory properties were discovered about 50 years ago and are currently exploited in catheters, stents, thermostat springs, glasses frames and dental braces. In these materials the lower temperature martensite phase can be deformed into any shape, then once heated it transforms back into the austenite phase and into its original shape.
The shape memory effect is closely related to another property found in metal alloys known as superelasticity or pseudoelasticity. The phenomenon is really not elasticity, but a large permanent deformation of the solid structure that can be reversed through the reversal of the phase transition, returning the material to its original shape. Here, the reversible stretching occurs in response to a mechanical load rather than a temperature change.
This ability to change solid phases with a mechanical force is being exploited in the design of a new type of ‘green’ refrigerator. ‘People have started to pay attention to the latent heat which is generated from these materials [superelastic alloys] during their [solid state] phase transformations and looking at using that in refrigerators or heat pumps,’ says engineer Jaka Tušek, from University of Ljubljana, Slovenia.
A phase change is important in conventional refrigeration too – where the condensation of a low pressure gas absorbs latent heat and causes the system to cool. Together with colleagues from the Technical University of Denmark, Tušek is trying to take advantage of the latent heat released and absorbed in a solid state phase transformation created by the application and removal of mechanical strain. He thinks the reversible cycling of the austenite to martensite transition in Nitinol potentially provides a more efficient cooling method.5
The idea for this so-called elastocaloric refrigerator also stems from recent developments in magnetocaloric refrigeration, where another phase change is being exploited – this one due to changes in a material’s magnetic state. BASF unveiled a magnetocaloric fridge in 2015, which exploits the latent heat produced when a magnetic field is applied to an iron-manganese material.
Tušek says the latent heat available from an austenite to martensite transformation is much greater than that available in magnetocaloric materials ‘Our prototype device demonstrated a kilowatt of cooling power per kg of material, so for a normal fridge having 50W of cooling power, you would need something like less than 100g of elastocaloric material,’ suggests Tušek. ‘In a couple of years we should have a market-ready application.’
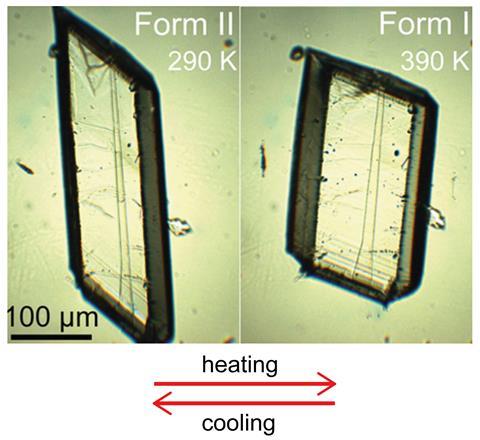
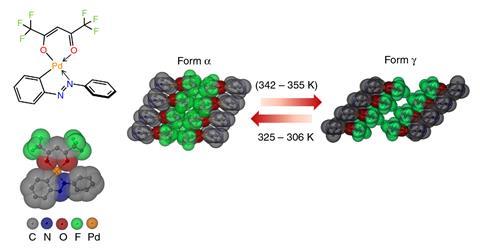
Naumov has recently discovered his organic materials will also undergo phase changes due to mechanical stress. Simply poking some crystals can induce martensitic phase transitions in organic materials – a process that mirrors the superelastic behaviour exploited in Nitinol heat pumps.6 He has called this the mechanosalient effect. Whether it may be possible to harness this phenomenon in organic materials for refrigeration, as Tušek is doing in alloys, is still to be seen, but Naumov says, ‘the idea is interesting’.
Organosuperelasticity
Superelastic properties in organic materials have been reported by Japanese coordination chemist Satoshi Takamizawa at Yokohama City University. ‘I decided to start screening single crystals by trying to crystallise all the chemical reagents stored in my laboratory,’ he says. He found that crystals of terephthalamide (C8H8N2O2) could be made to bend under mechanical pressure, due to an induced martensitic phase transition. When the pressure was released, the phase reverted to its original structure. Takamizawa was able to repeat this 100 times without fatigue.7 ‘Currently, we have about a dozen examples of different [organic superelastic] compounds in my laboratory and the number continues to increase,’ Takamizawa adds.
In superelasticity mechanical forces trigger the phase changes, but for a material to show shape-memory behaviour the phase change needs to be able to be reversed due to a change in temperature. So have organic materials been found that are comparable to shape-memory alloys and show this type of behaviour? ‘It’s even more difficult than finding mere superelasticity,’ says Takamizawa.
There are already organic materials that have shape memory properties – so-called shape-memory polymers have been around since the 1980s. These are soft materials that are deformed through thermal relaxation of the polymer structure. This relaxation can then be reversed using heat, light or solvents that cause the polymer to re-establish cross-linkages in their chains and so return to their original form. But these materials are soft and recover their shape ‘rather feebly’ according to Takamizawa.
In 2016, Takamizawa identified an organic crystal with the desired shape memory properties. Rod-shaped tetrabutyl-n-phosphonium tetraphenylborate (PBu4BPh4) crystals showed powerful shape recovery along with easy deformability.8 When a force was applied the crystals could be bent at room temperature – even producing a crystal in a zigzag shape. On warming, the crystals spontaneously returned to a straight shape.
Searching for applications
Fascinating effects such as jumping crystals might keep lab members huddled around the microscope and chatting about it over coffee, but are they useful for anything else? Martensitic transitions in metals have given us shape memory alloys and possibly a new refrigeration method – but what about these similar phenomenon in organic materials? Naumov has been working hard to find applications.
One potential use is in artificial muscle and creating actuators for soft robotics – robots which are made completely from polymers with no inorganic parts. ‘These crystals could actually move polymers,’ suggests Naumov. He has created what he calls an all-in-one biomimetic hybrid actuator, containing thermosalient crystals of PHA. The PHA was crystallised in situ, embedded into a protein matrix and cast into plastic moulds. After three days of curing, the elastic orange films produced could be stimulated to move. When heated the films bent and on cooling straightened. The transition occurred at around 75°C or by using UV or blue light.9
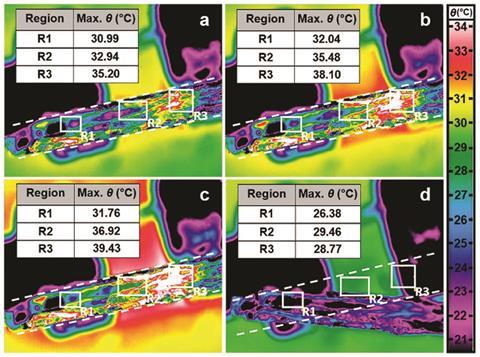
Naumov is also making use of these materials in organic electronic components and circuits. ‘The electric fuse as a simple demonstration of how you can use this organic crystal in electronics [and] shows the potential of these materials in future applications,’ he says. He has created a fuse that exploits the solid-solid phase transition of a thermosalient crystal, rather than the solid–liquid transition used in a conventional wire fuse. Naumov incorporated a silver coated 2,4,5-tetrabromobenzene crystal into a small circuit. Above a threshold level, the heat due to the electrical current flowing through the silver causes the crystal to undergo a phase transformation and move or explode, breaking the circuit. Naumov’s fuse, made with a 7mm long crystal, withstood a current of up to 170 mA.10
The practicality of designing devices with these kinds of solid state transitions in organic materials may not be easy though. Organic crystals are generally soft and don’t have the malleability that allow metals to be shaped or drawn into wires. To overcome this, Naumov’s group have been looking at ways to grow crystals using templates.
Whether these sorts of molecular materials can ultimately be utilised for their energy transducing properties, remains to be seen – but Takamizawa is optimistic about the sorts of innovations that might be achieved. Looking back on the history of functional materials, he says, ‘evolution occurs when organic materials take over’.
‘The huge advantage of organic materials is that there is an unlimited number of variations,’ muses Bernstein. ‘The fact that you can take organic materials and make very subtle changes by substituting chemical groups, it just makes it potentially much more versatile and expandable than working with metals.’
Rachel Brazil is a science writer based in London, UK
References
1 M Etter and A Siedle, J. Am. Chem. Soc., 1983, 105, 641 (DOI: 10.1021/ja00341a065)
2 P Naumov et al, Angew. Chem. Int. Ed., 2013, 52, 9990 (DOI: 10.1002/anie.201303757)
3 M Panda et al, Nat. Commun., 2014, 5, 4811 (DOI: 10.1038/ncomms5811)
4 M Panda et al, Angew. Chem. Int. Ed., 2017, 28, 8104 (DOI: 10.1002/anie.201702359)
5 K Engelbrecht et al, Nat. Energy, 2016, 1, 16134 (DOI: 10.1038/nenergy.2016.134)
6 D Karothu et al, J. Am. Chem. Soc., 2016, 138, 13298 (DOI: 10.1021/jacs.6b07406)
7 S Takamizawa and Y Miyamoto, Angew. Chem. Int. Ed., 2014, 53, 6970 (DOI: 10.1002/anie.201311014)
8 S Takamizawa and Y Takasaki, Chem. Sci., 2016, 7, 1527 (DOI: 10.1039/c5sC04057d)
9 S Sahoo et al, RSC Adv., 2014, 4, 7640 (DOI: 10.1039/c3rA46688d)
10 A Khalil, E Ahmed and P Naumov, Chem. Commun., 2017, 53, 8470 (DOI: 10.1039/c7cc04251e)










No comments yet