Recent high profile controversies haven’t deterred scientists from searching for one of research’s ultimate prizes: room temperature superconductors. Kit Chapman reports on the claims
In July 2023, the world became obsessed with superconductivity. Two pre-prints from a group in South Korea claimed that a copper-doped lead-apatite, dubbed LK-99 after its two proposers, Lee Sukbae and Kim Ji-Hoon, was a superconductor at room temperature and ambient pressure. The claims spread across social media, with both seasoned groups and amateur chemists trying to recreate the material. By August, a consensus was reached that LK-99 was yet another dead end, and not a superconductor at all.
The news followed a paper in Nature that proposed another room-temperature superconductor, this time only showing its properties at intense pressures, by Ranga Dias at the University of Rochester in the US. Yet Dias’ claims have now been retracted, and his data and academic reputation have been brought into question amid allegations of research fraud and plagiarism.
So how close are we to finding one of the remaining holy grails of modern chemistry?
Coming in from the cold
A superconductor is a material that shows no electrical resistance. This is an incredibly useful property: it could transform how electricity is transferred and stored, be used to create super-efficient electric motors and magnetic levitation trains, or help develop quantum computers. The problem is that materials typically only show this property at extremely cold temperatures, limiting their use to specialised magnets such as in MRI machines. To date, five different Nobel prizes have been awarded for superconductivity research, as researchers have gradually found materials that show superconductivity at warmer temperatures. The latest came in 1987, thanks to the discovery of the cuprate superconductors, metal oxides sandwiched between copper oxides.
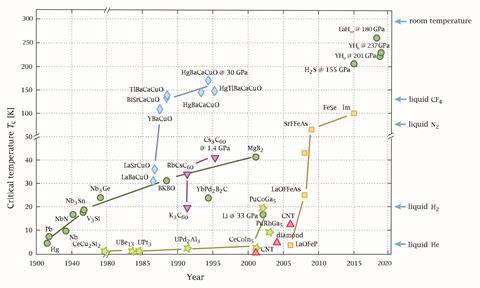
‘Room temperature superconductivity is absolutely possible,’ says Jorge Hirsch, a professor of physics at the University of California, San Diego, US. ‘And it will happen. There’s no question. When? That’s a big question. Before we had the cuprates, the highest critical temperature was 22K. After the cuprates, the highest critical temperature was 140K, it went up by a factor of six. Now we just need a factor of two to get to room temperature – there’s no intrinsic reason we won’t get there.’
Unfortunately, the cuprates are unlikely to be the answer. ‘Cuprates are very challenging to use in practical applications,’ says Graeme Ackland, professor of computer simulation at the University of Edinburgh, UK. ‘They’re ceramics, it’d be like trying to make wires out of chocolate biscuits.’ They are also poorly understood, as they don’t follow the standard model of superconductivity, BCS theory. And this is what made the LK-99 pre-print so tantalising: the jump isn’t outside of the realms of possibility, and without a concrete mechanism, it’s impossible to rule the material out completely. ‘No one can go away and take, say, rare earth-barium-copper-oxide,’ Ackland adds, ‘do a calculation and work out its superconducting temperature and be right to within 50K.’ You have to experiment.
LK-99 went viral when a video appeared to show the material levitating – suggesting the Meissner effect, the expulsion of a magnetic field from a material as it becomes a superconductor, causing it to repel weak magnetic fields. Unfortunately, this was likely due to ferromagnetism, which requires a single point of contact, instead of true levitation. Thanks to the efforts of social media sleuths and multiple research teams, LK-99’s celebrity turned out to be short-lived; although some researchers continue to investigate the material, the hype has gone and to date no successful attempt to replicate the finding has been published in peer-reviewed journals.
‘That claim had very little credibility from day one,’ says Hirsch. ‘I’m surprised it got so much attention … when I looked at the composition, I thought “Yeah, it’s not impossible that material could be a room temperature superconductor.” But there was really no good evidence. And that’s really a problem in the field. People are very excited about the idea, they jump to conclusions when they see anything that looks suggestive.’
This means that it’s likely there might be more false alarms such as LK-99 in the future, something that concerns Hirsch. ‘It’s damaging the field,’ he says. ‘I think it’s damaging the credibility, especially when it gets picked up in the media and a lot of publicity. And people that aren’t experts look at things and say “Wow, that looks right!” – it takes a lot of bandwidth, and in the end, doesn’t amount to anything.’
But there is an alternative approach that could offer a tantalising route to superconductivity: high pressure. Sadly, it’s also seen its share of controversy.
Under pressure
In 2019, Mikhail Eremets, a high pressure chemist at the Max Planck Institute for Chemistry in Mainz, Germany, reported superconductivity in lanthanum hydride at a temperature of 250K, a mere 40K below room temperature. The catch was that it was at a pressure of around 170 gigapascals (GPa), roughly seven times the pressure inside the Sun’s core. This was achieved by squeezing the material inside a diamond anvil cell, a feat only a handful of labs in the world are capable of replicating. ‘That’s an astonishing pressure,’ says Ackland. ‘Hydrogen will readily diffuse into your diamond and break the cell. That’s the reason relatively few groups can do it – you can’t just have any old diamond anvil cell, you really have to know what you’re doing.’
Eremets is one such researcher. ‘We’re on the verge of achieving room temperature superconductivity,’ he says. ‘There are a number of predicted compounds, such as magnesium hydride, lanthanum hydride, cerium hydride and lithium-magnesium-hydride systems, where the calculated critical temperatures approach or exceed room temperature.’
The reason why superconductivity is expected to appear at high pressure comes back to chemistry, explains Ackland. ‘If you have two hydrogen atoms, they want to stick together and make a molecule. In order to get superconductivity, you have to break that covalent bond. You either immerse it in a metal, so there are other electrons flying around, or you put it under extremely high pressure, so they share electrons between all, rather than with pairs. If you break the hydrogen into individual atoms, you can generate the phonons [excitation of atoms in condensed matter] that hold the electrons together. That’s what gives you the superconductivity.’ This is why much of the research focuses around group 3 hydrides. ‘If you want to break the chemical bond, you do that by immersing it in a lot of other electrons,’ says Ackland. ‘So, what material will release the largest electron density?’
The challenge with working in a black-box environment such as a diamond anvil cell is that replication is far harder than with LK-99 – and led to arguably the most controversial chapter in superconductivity’s history. In 2020, Dias’s team claimed to have discovered a carbonaceous sulfur hydride that showed superconductivity at 287K – 14°C – albeit at an incredible 267GPa. Despite being hard to verify, if the claim was true, it would have been the breakthrough the community was searching for. It was a result that catapulted Dias to Time magazine’s ‘100 Next’ most influential figures in the world.
The paper was immediately challenged by Hirsch, an opponent of hydrides who remains unconvinced (largely against the scientific consensus) of their superconductivity. To him, the paper’s plot showing electrical resistance seemed far too sharp. Within a week, he and Frank Marsiglio of the University of Alberta, Canada, had co-authored a letter to Nature. At the time, the community still supported Dias; Hirsch had been critical of hydrides before, surely he was wrong.
Hirsch persisted, demanding the group’s original data. Soon, it emerged one of Dias’ co-authors had inappropriately manipulated data on a paper 12 years earlier, which was then retracted. Other researchers began to question the Dias work.
In November 2021, Dias released his raw data. Once again, Hirsch investigated, this time with Dirk van der Marel of the University of Geneva, Switzerland. Their conclusion was even more dramatic: the results were not simply wrong, they were falsified. In 2022, Dias’ paper was retracted by Nature over questions ‘raised regarding the manner in which the data in this paper have been processed and analysed’.
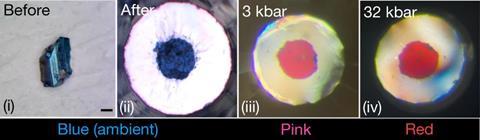
Dias denied that he, or any member of his team, committed research fraud. In March 2023, he publisheda second claim for a room temperature superconductor, this time nitrogen-doped lutetium hydride, at much lower pressures. Far from being heralded, it immediately faced questions from researchers. It was ‘like winning the lottery to discover a room-temperature superconductor, and they’re claiming to have done it multiple times,’ James Hamlin, a physicist at the University of Florida, US, said to Undark in 2023. ‘It just does not make sense.’ Hamlin’s allegations didn’t end there: he sent an anonymous critique of the work to Nature about the reliability of the paper’s electrical resistance data.
‘There was a signature of that material: it went from blue to red under pressure,’ Ackland explains. ‘Which meant that anybody trying to reproduce the work could fairly convincingly verify that they got the same material.’ Although the colour change didn’t have anything to do with superconductivity, this and the comparatively low pressure allowed other labs to accurately replicate the effect. None of them were able to do so.
In a post-publication review, four independent experts recommended the new paper was retracted. Soon after, all of the paper’s authors including his long term collaborator, Ashkan Salamat of the University of Nevada, US, – but not Dias and two of his students – signed a letter requesting that the paper be retracted as it did not ‘accurately reflect the provenance of the investigated materials, the experimental measurements undertaken and the data-processing protocols applied’. Dias’ alma mater, Washington State University, also launched an investigation into allegations he had plagiarised more than 20% of his PhD thesis.
To an outsider, such retractions and unverified claims may cast a shadow over the field
In April 2024, a 10-month investigation by Rochester concluded that, based on the preponderance of evidence, Dias had committed numerous instances of research misconduct. This included manipulating data in a ‘blatant and significant departure from accepted practices within the research community’, which ‘clearly constitutes intentional data fabrication’, in addition to plagiarism that ‘clearly rises to the level of intentional’. The university investigation concluded that Dias ‘cannot be trusted’ and their findings ‘are tantamount to a recommendation of termination [of his position]’.
‘There’s incontrovertible evidence that there was a lot of faking of the figures,’ says Ackland. And there are still questions that aren’t answered. ‘There’s a figure that’s a fairly minor detail in the paper, but appears faked. Who made it? This doesn’t seem like a difficult question [but] it seems fundamental.’
There’s little doubt that the Dias papers have cast a shadow over the field. However, Eremets remains convinced that, far from being a problem, it’s a sign science worked. ‘To an outsider, such retractions and unverified claims may cast a shadow over the field,’ Eremets agrees. ‘But this is the remarkably self-correcting process of science, which does not fear even fraud.’
Even so, it leaves a big question: where do we go next?
Resistance is futile
The consensus within the community is that, even if Dias’ claims aren’t credible, hydrides are the way forward. Hydride research is growing, with around 50 groups worldwide now working on the problem. ‘With the accumulated knowledge at high pressures, the focus is shifting to the search for new hydrides at lower and ambient pressures,’ Eremets says.
These are not likely to be at room temperature – the current models predict 100K. They do, however, represent a major leap forward. ‘New superconductors with a critical temperature above the boiling point of liquid nitrogen could significantly enhance applications, complementing the cuprates and overcoming their inherent limitations,’ says Eremets. And, if a material is confirmed to be a room-temperature superconductor at high pressure, it may be possible to design a material that retains that property at a lower pressure – it would give scientists a road map to work toward.
I suspect we’re looking at a fairly short period of time, or never,
Eremets is also confident that, even if hydrides aren’t likely to lead to room-temperature superconductors directly, the knowledge we’ve gained is still of use. ‘Numerous theoretical calculations demonstrate high temperatures … in carbon-cage network structures,’ he says. ‘The successful synthesis of other light-element based systems, such as metal-boron-carbon framework crystal lattices, show very promising results.’
Yet the ramifications of the shadows cast on superconductivity continue. ‘The field is not in good shape for many reasons,’ Hirsch says. ‘The credibility has been damaged by LK-99, and the hydrides even more.’ While these are isolated cases, Hirsch argues that it shows the entire community – including theorists and experimentalists – need to take a step back and critically appraise the situation. He also argues that journals should be more robust in making sure raw data are available to other researchers, so claims can be verified (or potential pitfalls identified) quickly.
And Hirsch remains unconvinced hydrides are superconductors at all. ‘In my view, that’s completely wrong. When you look at the big picture, there’s no evidence at all that light elements favour superconductivity other than the claims for the hydrides, which I don’t think are real. I see all the signals that people see, and I think they’re due to other things, not superconductivity.’ The challenge is that, as the experiments are performed in a diamond anvil cell, it’s hard to prove his case. ‘I look at the experimental evidence more critically than other people,’ Hirsch says. ‘I find lots and lots of flaws with it.’
Hirsch advocates a different approach instead. ‘What is it that cuprates and conventional superconductors, such as magnesium diboride, the highest temperature superconductor in that class, have in common? Negative ions. You could make structures with negative ions in close proximity; it’s very difficult in practice, because it creates an unstable situation, so you’d need to stabilise the lattice by having positive counter-ions off the plane. If you can stabilise those structures in creative ways, I think you’re going to find a room temperature superconductor.’
Eremets considers Hirsch’s actions ‘destructive’. ‘He tends to focus on isolated details of experiments, casting doubt on [hydride] superconductivity as a whole … his methods, including the use of unverified models, selective data manipulation, and flawed analyses, represent a departure from ethical scientific practice.’ Hirsch points out that his papers analysing the experimental data reported by Eremets and others are published in peer-reviewed scientific journals that do not endorse publication of flawed analyses that depart from ethical scientific practice.
Once again, the superconductivity community has found itself in a war of words, of claims and counterclaims – and one that’s only likely to be resolved with the next breakthrough. Given the stakes – Nobel prizes and a trillion-dollar technology – it’s unlikely the scrutiny is going to fade. The good news is that the community is convinced it’s on the verge of cracking the problem. ‘I suspect we’re looking at a fairly short period of time, or never,’ says Ackland. ‘I feel that it’s one more clever step that’s needed, rather than searching forever.’
Kit Chapman is a science journalist based in Falmouth, UK
Article updated 24 April 2024 to include Hirsch’s response to Eremets in the penultimate paragraph.

















No comments yet